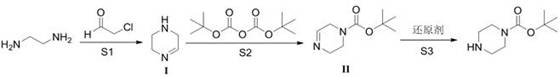
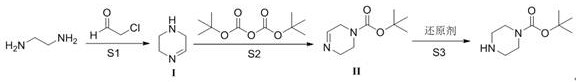
Preparation method of N-t-butyloxycarbonyl piperazine
A technology of tert-butoxycarbonylpiperazine and solvent medium, which is applied in the field of synthesis of pharmaceutical intermediates, and can solve problems such as high price, low product yield, and difficult preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add ethylenediamine (18.1 g, 1.1 eq) to a 500 mL three-necked flask, add 50 mL of methanol, start stirring, cool the reaction system below 10°C, add 40% chloroacetaldehyde aqueous solution (53.6 g, 1 eq) dropwise, drop The addition time is about 1 hour, after the addition is completed, the temperature is raised to 20-30°C, and the reaction is carried out for 2 hours. After reaction at ℃ for 3 hours, dichloromethane (50 mL + 20 mL) was added to extract twice after the reaction was completed, the organic phases were combined and concentrated to obtain 19.6 g of compound I with a yield of 85.4% and a purity of 97.7% by gas chromatography;
[0025] Compound I (50 g, 1 eq) was added to a 500 mL three-neck flask, 250 mL of methanol was added, and BocO 2 Acid anhydride (142.9 g, 1.1 eq), the dropwise addition time is about 1 hour, after the dropwise addition is completed, react at 25°C for 2 hours, the TLC control reaction is completed, add sodium borohydride (11.3 g, 0.5 eq) ...
Embodiment 2
[0027] Add ethylenediamine (20 g, 1 eq) to a 500 mL three-necked flask, add 50 mL of methanol, start stirring, cool the reaction system to below 10°C, add 40% aqueous solution of chloroacetaldehyde (65.3 g, 1 eq) dropwise, The dropping time is about 1 hour. After the dropping is completed, the temperature is raised to 20-30°C, and the reaction is performed for 2 hours. At a temperature of 20-30°C, 60 g of aqueous sodium hydroxide solution (w=30%) is added dropwise. React at 45°C for 3 hours. After the reaction is completed, dichloromethane (50 mL + 20 mL) is added to extract twice, and the organic phases are combined and concentrated to obtain 20.6 g of compound I with a yield of 73.7% and a purity of 97.2% by gas chromatography;
[0028] Compound I (50 g, 1 eq) was added to a 500 mL three-neck flask, 250 mL of methanol was added, and BocO 2 Acid anhydride (129.9 g, 1 eq), the dropwise addition time is about 1 hour, after the dropwise addition is completed, react at 25°C for 2...
Embodiment 3
[0030] Add ethylenediamine (20 g, 1.1 eq) to a 500 mL three-necked flask, add 50 mL of methanol, start stirring, cool the reaction system to below 10 °C, add dropwise 40% chloroacetaldehyde aqueous solution (59.4 g, 1 eq), The dropping time is about 1 hour. After the dropping is completed, the temperature is raised to 20-30°C, and the reaction is carried out for 2 hours. At 20-30°C, 60 g of aqueous sodium hydroxide solution (w=30%) is added dropwise. React at 30°C for 3 hours. After the reaction is completed, dichloromethane (50 mL + 20 mL) is added to extract twice, and the organic phases are combined and concentrated to obtain 18.8 g of compound I with a yield of 67.4% and a purity of 97.6% by gas chromatography;
[0031] Add compound I (30 g, 1 eq) into a 500 mL three-neck flask, add 250 mL of methanol, and add BocO 2 Acid anhydride (85.7 g, 1.1 eq), the dropwise addition time is about 1 hour, after the dropwise addition is completed, react at 35°C for 2 hours, the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com