Production of high purity lithium carbonate from brines
A lithium carbonate, brine technology, applied in the direction of lithium carbonate;/acid carbonate, etc., can solve the problems of waste of resources, technical complexity, high cost, etc., to achieve improved productivity, low technical complexity, and shortened time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0168] A) Removal of calcium ions, magnesium ions and strontium ions by precipitation
[0169] To 1 l of brine (37 g / l of Ca 2+ , 3.7g / l of Mg 2+ , 2.3g / l of Sr 2+ , 65g / l Na + and 140ppm Li + (0.014g / l)) adding 0.32l of Na 2 CO 3solution (400g / l, 129.3g) and 0.05l of NaOH (1000g / l, 48.8g). The brine and precipitating agent are mixed here at a stirring rate of 350 rpm. The dispersion formed was filtered off via a filter funnel at a pressure of 2 bar. Purified brine contains Ca at a concentration below 20ppm 2+ 、Sr 2+ and Mg 2+ and 140ppm Li + And has a pH of 11.
[0170] B) Removal of Ca by chelating resins containing aminomethylphosphonic acid groups 2+ 、Sr 2+ and Mg 2+
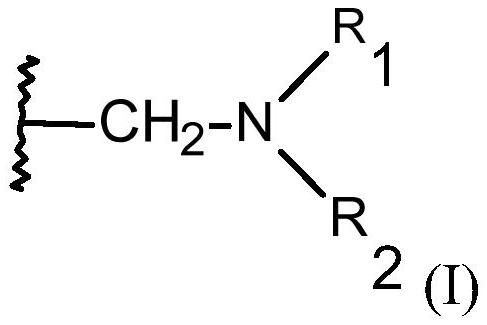
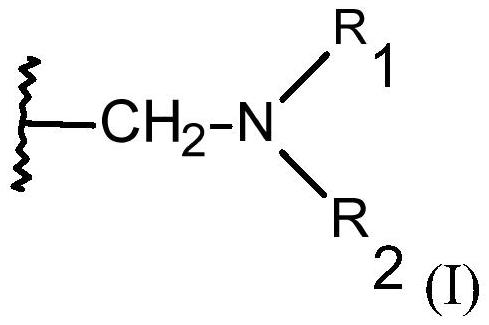
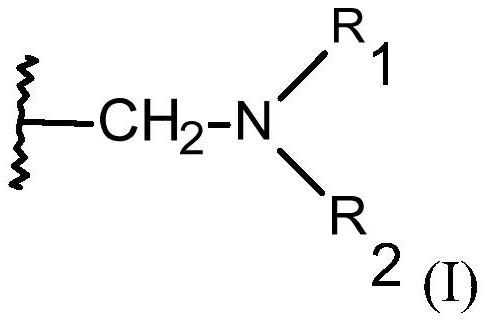
[0171] The macroporous monodisperse chelating resin that contains the functional group with structural element (I) that vector cylinder fills 50ml, wherein R 1 and R 2 independently of each other = -CH 2 PO(OX 1 ) 2 、-CH 2 PO(OH)OX 2 or hydrogen, where both cannot be hydrogen at ...
example 2
[0178] A) Removal of calcium ions, magnesium ions and strontium ions by precipitation
[0179] To 1 l of brine (37 g / l of Ca 2+ , 3.7g / l of Mg 2+ , 2.3g / l of Sr 2+ , 65g / l Na + and 140ppm Li + (0.014g / l)) add 0.32ml of Na 2 CO 3 solution (400g / l, 129.3g) and 0.05l of NaOH (1000g / l, 48.8g). The brine and precipitating agent are mixed here at a stirring rate of 350 rpm. The dispersion formed was filtered off via a filter funnel at a pressure of 2 bar. Purified brine contains Ca at a concentration below 20ppm 2+ 、Sr 2+ and Mg 2+ and 140ppm Li + And has a pH of 11.
[0180] B) Removal of Ca by chelating resins containing iminodiacetic acid groups 2+ 、Sr 2+ and Mg 2+
[0181] The macroporous monodisperse chelating resin that contains the functional group with structural element (I) that vector cylinder fills 50ml, wherein R 1 and R 2 independently of each other = -CH 2 COOX or hydrogen, but R 1 and R 2 cannot both be hydrogen at the same time, and X is hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com