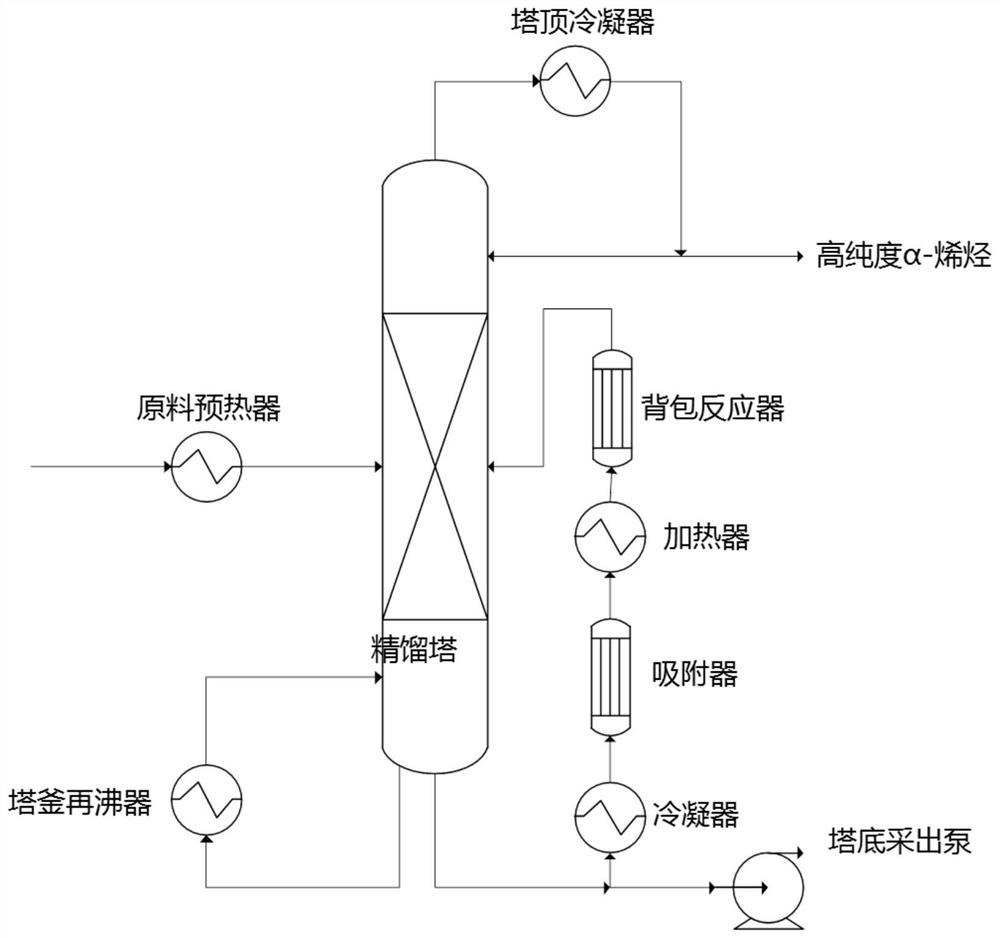
Method for producing high-purity alpha-olefin by adopting backpack type reactive distillation device
A high-purity, olefin technology, applied in separation methods, chemical instruments and methods, hydrocarbon purification/separation, etc., can solve problems such as lack of high-purity, improve catalytic efficiency and service life, achieve single-column separation, adsorption capacity big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: ZnO-ZrO 2 / Synthesis of ZSM molecular sieve
[0042] Under stirring conditions, 2.85g water glass (Na 2 SiO 3 9H 2 (2, molecular weight 284g / mol), 300g aluminum isopropoxide, 350g deionized water join in 1.83g n-butylamine and mix;
[0043] After mixing evenly, add 5.95g of Zn(NO 3 ) 2 ·6H 2 O, 0.68g of Zr(NO 3 ) 4 ·5H 2 O. 2.30g of glycolic acid, put the beaker in a water bath and stir at 80°C to fully dissolve it. After the solid is completely dissolved, transfer it to an oven at 170°C for hydrothermal reaction for 36 hours. After the reaction, evaporate to obtain viscosity Gel, put it into a muffle furnace after grinding, and keep it at 500°C for 4h to get ZnO-ZrO 2 / ZSM molecular sieves as heterogeneous catalysts.
[0044] Similarly, repeat the above preparation process without adding Zn(NO 3 ) 2 ·6H 2 O and Zr(NO 3 ) 4 ·5H 2 O, to obtain silica-alumina molecular sieves that do not contain active double metal oxides as adsorbents.
Embodiment 2
[0045] Example 2: ZnO-ZrO 2 / Synthesis of ZSM molecular sieve
[0046] Under stirring condition, 2.84g water glass (Na 2 SiO 3 9H 2 (2, molecular weight 284g / mol), 370g aluminum nitrate, 560g deionized water join in 1.5g n-butylamine and mix;
[0047] After mixing evenly, add 3.56g of Zn(NO 3 ) 2 ·6H 2 O, 0.68g of Zr(NO 3 ) 4 ·5H 2 O. 1.52g of glycolic acid, put the beaker in a water bath and stir at 90°C to make it fully dissolve. After the solid is completely dissolved, transfer it to an oven at 160°C for hydrothermal reaction for 24 hours. After the reaction, evaporate to obtain viscosity Gel, put it into the muffle furnace after grinding, and keep it at 550°C for 3h to get ZnO-ZrO 2 / ZSM molecular sieves as heterogeneous catalysts.
[0048] Similarly, repeat the above preparation process without adding Zn(NO 3 ) 2 ·6H 2 O and Zr(NO 3 ) 4 ·5H 2 O, to obtain silica-alumina molecular sieves that do not contain active double metal oxides as adsorbents.
Embodiment 3
[0049] Example 3: ZnO-ZrO 2 / Synthesis of ZSM molecular sieve
[0050] Under stirring conditions, 2.1g of silica sol (SiO 2 ·8H 2 (2), 400g aluminum isopropoxide, 820g deionized water join in 3.0g di-n-propylamine and mix;
[0051] After mixing evenly, add 11.58g of Zn (NO 3 ) 2 ·6H 2 O, 1.02g of Zr(NO 3 ) 4 ·5H 2 O. 3.04g of glycolic acid, put the beaker in a water bath and stir at 80°C to fully dissolve it. After the solid is completely dissolved, transfer it to an oven at 180°C for hydrothermal reaction for 48 hours. After the reaction, evaporate to obtain viscosity Gel, put it into the muffle furnace after grinding, and keep it at 550°C for 3h to get ZnO-ZrO 2 / ZSM molecular sieves as heterogeneous catalysts.
[0052] Similarly, repeat the above preparation process without adding Zn(NO 3 ) 2 ·6H 2 O and Zr(NO 3 ) 4 ·5H 2 O, to obtain silica-alumina molecular sieves that do not contain active double metal oxides as adsorbents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com