Injectable skin filler and preparation method thereof
A technology of dermal fillers and dispersants, which is applied in the field of medical cosmetology, can solve the problems of cumbersome preparation process and achieve the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
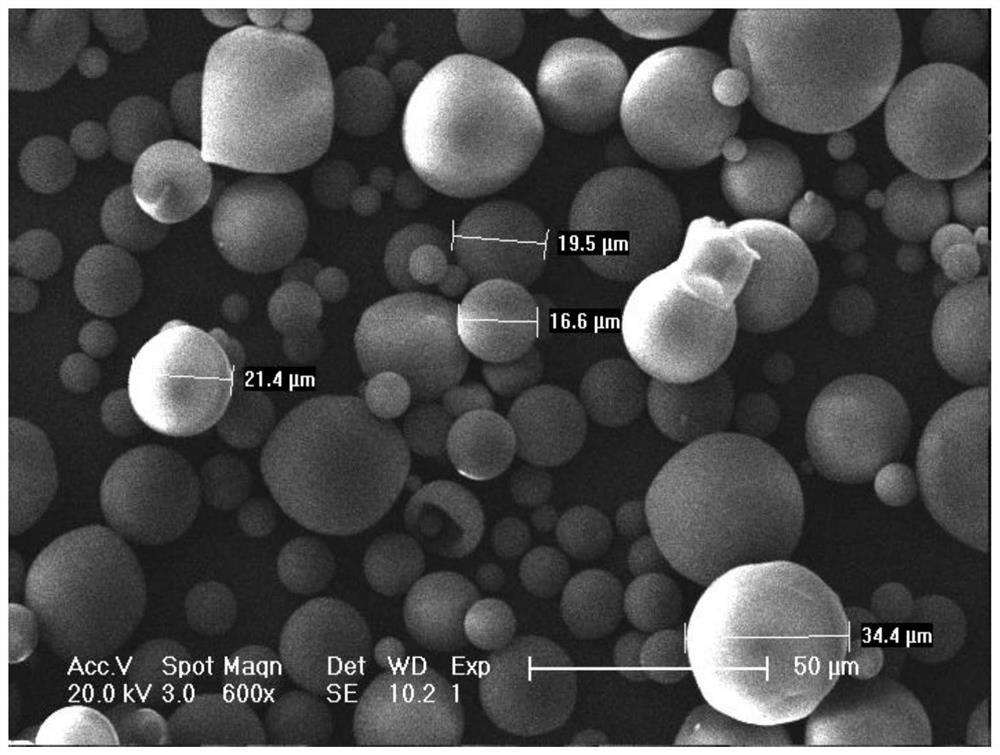
[0051] An injectable dermal filler, comprising the following raw materials in mass percentage: poly-L-lactic acid 35.6%, sodium hyaluronate 23.7%, mannitol 40.7%; the dermal filler is wrapped in poly-L-lactic acid by sodium hyaluronate and mannitol Particles formed on the surface of lactic acid.
[0052] The specific preparation method is as follows:
[0053] Step 1: Set the intrinsic viscosity to 0.9m 3 Sodium hyaluronate / kg is dissolved in water to prepare a sodium hyaluronate aqueous solution, wherein the mass percentage of sodium hyaluronate and water in the sodium hyaluronate aqueous solution is 1%. Add 10.3 g of mannitol into the sodium hyaluronate aqueous solution, and stir to dissolve to obtain the first mixed liquid; wherein the first mixed liquid is the water phase.
[0054] Step 2: Weigh 9 g of poly-L-lactic acid with an intrinsic viscosity of 1.5 dL / g, dissolve it in 100 mL of dichloromethane, and prepare a second mixed liquid; wherein the second mixed liquid is ...
Embodiment 2
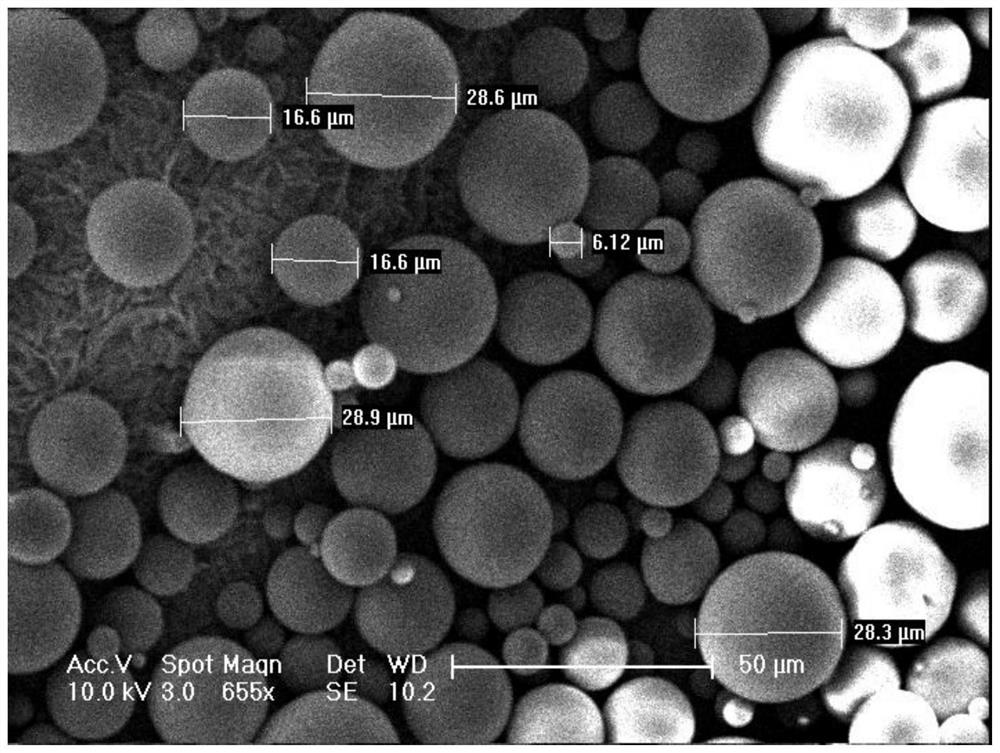
[0061] An injectable dermal filler, comprising the following raw materials in mass percentage: 48.0% poly-L-lactic acid, 20.0% sodium hyaluronate, and 32.0% glycine; the dermal filler is made of sodium hyaluronate and glycine wrapped on the surface of poly-L-lactic acid formed particles.
[0062] The specific preparation method is as follows:
[0063] Step 1: Set the intrinsic viscosity to 2.1m 3 Sodium hyaluronate / kg is dissolved in water to prepare a sodium hyaluronate aqueous solution, wherein the mass percentage of sodium hyaluronate and water in the sodium hyaluronate aqueous solution is 0.5%. Add 4.0 g of glycine to the sodium hyaluronate aqueous solution, and stir to dissolve to obtain the first mixed solution; wherein the first mixed solution is the water phase.
[0064] Step 2: Weigh 6 g of poly-L-lactic acid with an intrinsic viscosity of 1.8 dL / g, dissolve it in 80 mL of chloroform, and prepare a second mixed liquid; wherein the second mixed liquid is an oil phase...
Embodiment 3
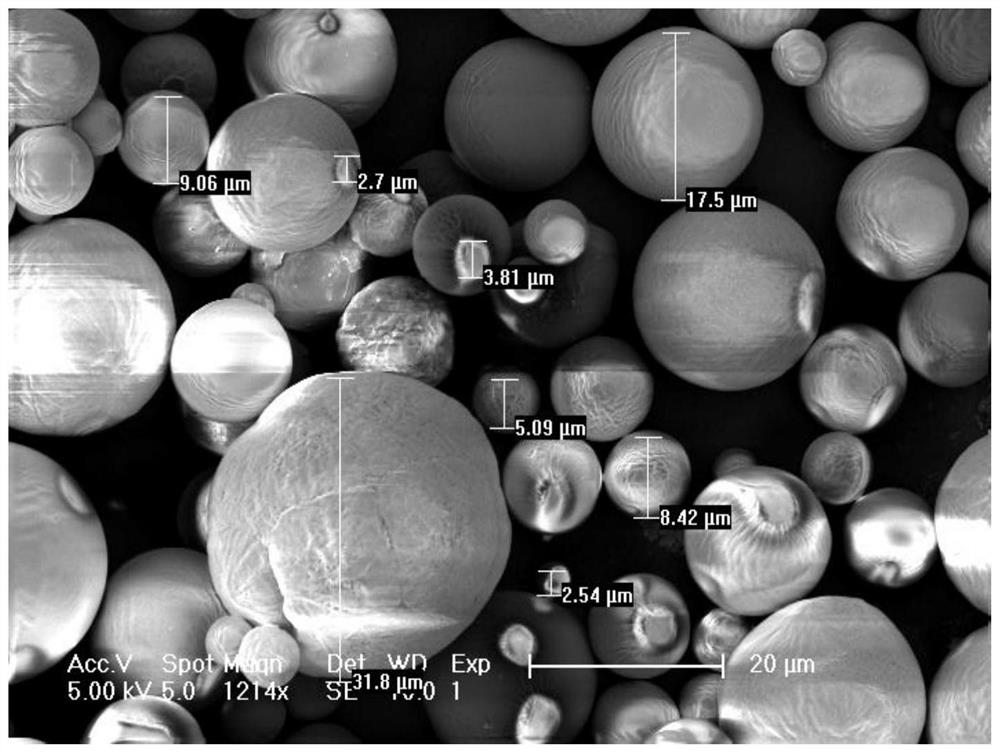
[0071] An injectable dermal filler, comprising the following raw materials in mass percentage: 5.3% polycaprolactone, 3.4% sodium hyaluronate, 3.2% alanine and 88.1% water; the dermal filler is made of polycaprolactone in Suspension formed in sodium hyaluronate and alanine.
[0072] The specific preparation method is as follows:
[0073] Step 1: Set the intrinsic viscosity to 2.6m 3 Sodium hyaluronate / kg is dissolved in water to prepare a sodium hyaluronate aqueous solution, wherein the mass percentage of sodium hyaluronate and water in the sodium hyaluronate aqueous solution is 0.8%. Add 5.4g of alanine into the sodium hyaluronate aqueous solution, and stir to dissolve to obtain the first mixed solution; wherein the first mixed solution is the water phase.
[0074] Step 2: Weigh 9 g of polycaprolactone with an intrinsic viscosity of 1.6 dL / g, dissolve it in 90 mL of dichloromethane, and prepare a second mixed liquid; wherein the second mixed liquid is an oil phase.
[0075...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com