Leptin receptor affinity peptide and application thereof
An affinity peptide and receptor technology, applied in the field of biomedicine, can solve the problems of inability to exert differentiation and repair, achieve good tissue damage repair effect, high affinity, and improve the effect of recruitment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
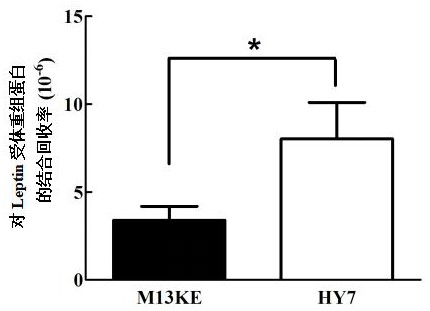
[0056] This example uses phage display technology to screen for affinity polypeptides that specifically bind to the Leptin receptor
[0057] Phage random heptapeptide display library was purchased from NEB Company, 100 µl, titer 1×10 13 pfu / ml. Stored in TBS buffer (50mM Tris-HCl, 150mM NaCl[PH7.5]) containing 50% glycerol, the storage capacity is 1.28×10 9 a transformant. Escherichia coil ER2738 is the host strain of this peptide library.
[0058] target protein coating
[0059] Dilute the Leptin receptor recombinant protein with coating buffer (0.1M sodium bicarbonate buffer [pH value 8.6]) to a final concentration of 100µg / ml, add 100µl to one well of the microtiter plate to ensure that the plate is wet, Incubate overnight at 4°C in a humid chamber.
[0060] affinity screening
[0061] The next day, pour off the coating solution in the ELISA plate, pat off the residual liquid on a clean absorbent paper, and add 200 µl of blocking solution (0.1M NaHCO 3 , 5mg / ml BSA [...
Embodiment 2
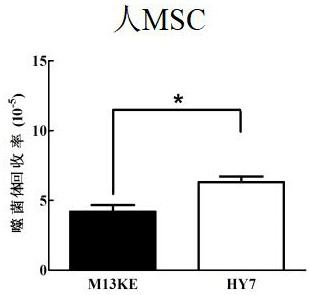
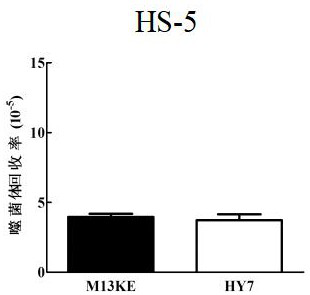
[0072] Example 2 HY7 can specifically bind to human mesenchymal stem cells
[0073] 1. Detection of the binding ability of HY7 phage monoclonal to human mesenchymal stem cells
[0074] Refer to the method in Example 1 to amplify HY7 phage monoclonal and M13KE negative control phage, and titrate. HY7 phage monoclonal and M13KE negative control phage were added to human mesenchymal stem cell well plates pre-cultured to about 80% confluence, and an irrelevant human bone marrow stromal cell line HS-5 was set as a control. Bind and elute according to the phage screening steps to obtain the HY7 phage eluate bound to the target protein and the M13KE negative control phage eluate respectively, and titrate the two according to the phage titration method to compare the binding of the two to the target molecule. The results show that HY7 can specifically bind to the surface of MSC cells, but not to the control cell HS-5, Figure 2A with Figure 2B It is a schematic diagram of the bind...
Embodiment 3
[0079] Example 3 CBD-affinity peptides can be bound to collagen materials for lung injury repair
[0080] 1. CBD-affinity peptide can be bound to collagen material
[0081] In order to avoid that the affinity peptide cannot effectively reside on the biological material and maintain an effective concentration caused by the simple adsorption method, which will affect the function of the affinity peptide. In the present invention, while synthesizing the affinity polypeptide, a short peptide CBD (the sequence is specifically TKKTLRT) with collagen-specific binding ability is introduced, synthesized at the N-terminus of the affinity polypeptide, and connected with the affinity polypeptide by a linker, the sequence is specific for GGG-K-FITC. In order to evaluate whether the CBD-affinity peptide can effectively bind to the collagen material, FITC-labeled CBD-HY7 was synthesized at the same time, and for the convenience of comparison, FITC-labeled CBD misconstructed control (sCBD-HY...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com