Thioxanthone visible light initiator, and preparation method and application thereof
A technology of thioxanthone and visible light, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
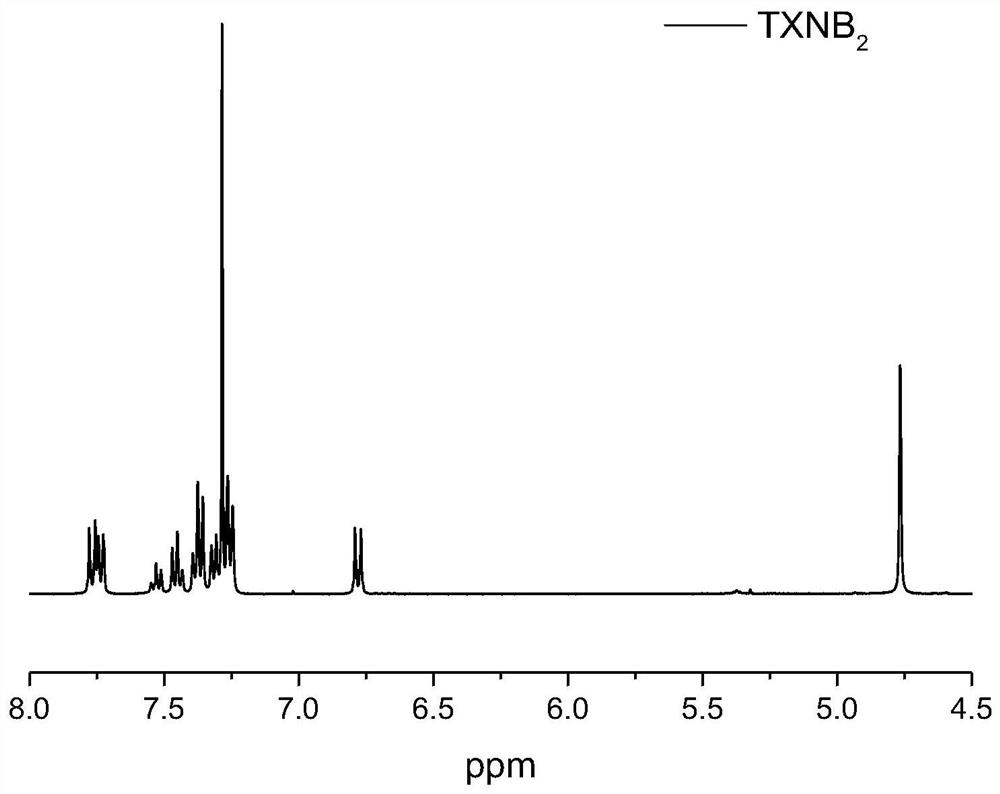
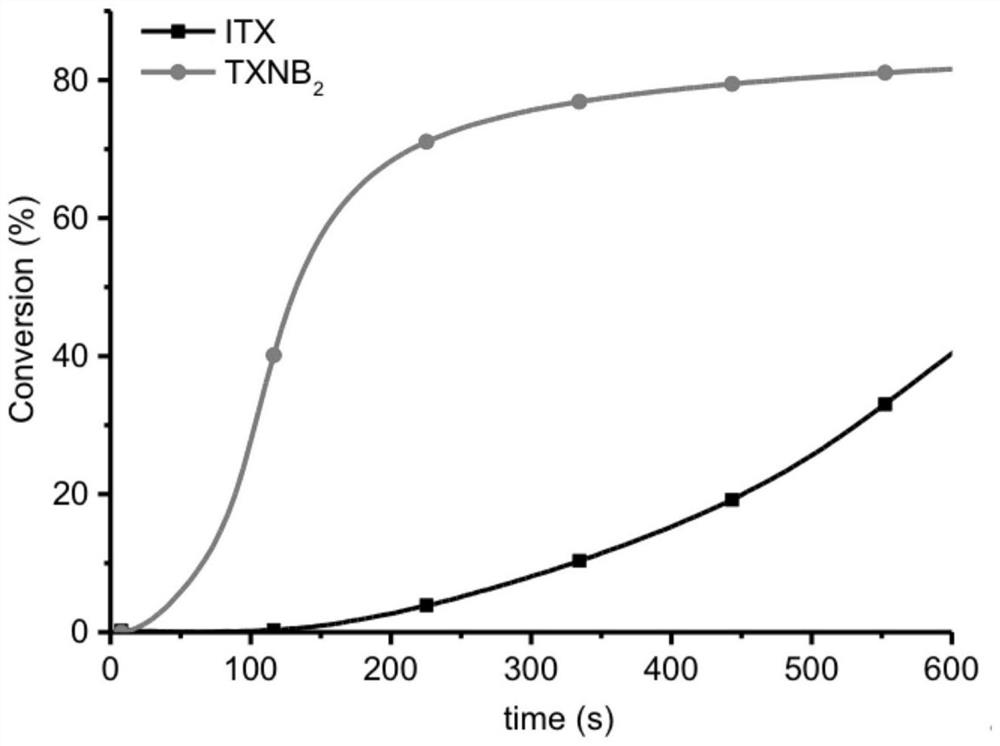
[0027] Preparation N, N-two (benzyl) thioxanthone photoinitiator (abbreviation TXNB 2 )
[0028] 2-aminothioxanthone (3.41g, 15.0mmol), benzyl chloride (3.89g, 30.75mmol), anhydrous potassium carbonate ( 4.67g, 33.825mmol), N,N-dimethylformamide 35mL, heated to 145°C under stirring, after 0.5h, TLC detected that the reaction was complete. Filter, concentrate, wash with water, dry, dissolve in dichloromethane, and reprecipitate in petroleum ether to obtain 5.07 g of pure product with a yield of 83%.
[0029] 1 H NMR (CDCl 3 , 400MHz) δppm (such as figure 1shown): 4.76(s, 4H), 6.75-6.80(d, J=8.80Hz, 2H), 7.22-7.27(m, 4H), 7.30-7.34(m, 2H), 7.34-7.40(m, 4H ), 7.42-7.48(m, 2H), 7.50-7.56(t, J=7.2Hz, 1H), 7.71-7.79(m, 4H).
Embodiment 2
[0031] Preparation of N,N-di(benzyl)thioxanthone photoinitiator
[0032] 2-aminothioxanthone (3.41g, 15.0mmol), benzyl chloride (4.08g, 32.25mmol), pyridine (3.19g, 40.31mmol), toluene 68mL, heated to 100°C under stirring, after 12h, TLC detected that the reaction was complete. Filter, concentrate, wash with water, dry, dissolve in dichloromethane, and reprecipitate in petroleum ether to obtain 5.61 g of pure product with a yield of 92%. The obtained pure product was confirmed to be N,N-di(benzyl)thioxanthone by proton nuclear magnetic resonance spectrum data.
Embodiment 3
[0034] Preparation of N,N-di(benzyl)thioxanthone photoinitiator
[0035] 2-Aminothioxanthone (3.41g, 15.0mmol), benzyl chloride (4.18g, 33.0mmol), sodium carbonate (4.37g , 41.25mmol), xylene 40mL, heated to 120°C under stirring, after 10h, TLC detected that the reaction was complete. Filter, concentrate, wash with water, dry, dissolve in dichloromethane, and reprecipitate in petroleum ether to obtain 5.68 g of pure product with a yield of 93%. The obtained pure product was confirmed to be N,N-di(benzyl)thioxanthone by proton nuclear magnetic resonance spectrum data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com