Application of ripasudil in preparation of medicine for treating bacterial infection
A technology for risudil and bacterial infection, applied in the field of medicine to achieve the effect of improving organ damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
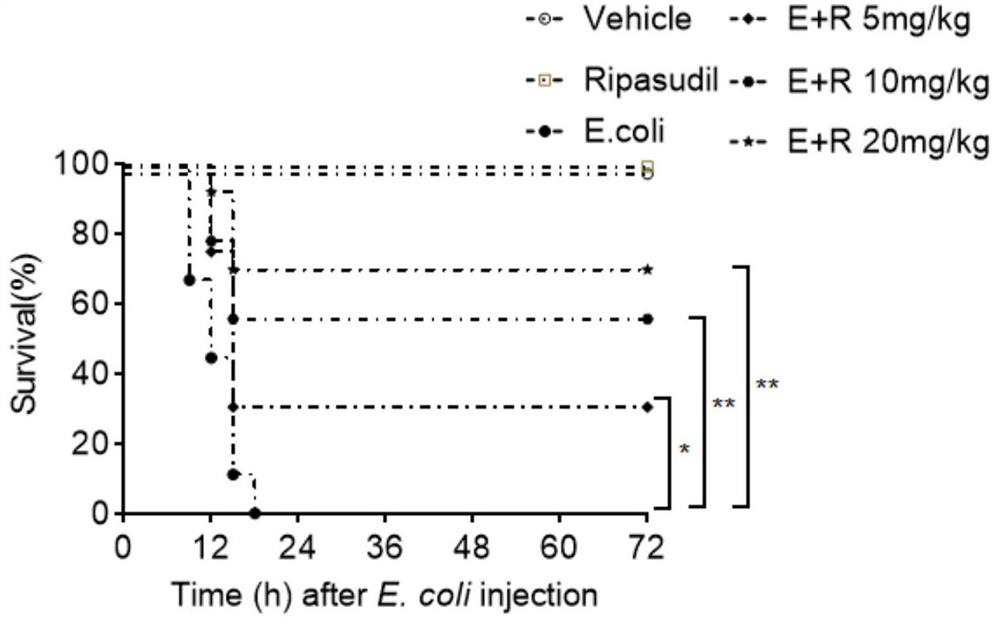
[0054] In order to verify the role of lisudil in the treatment of E.coli infection, E.coli was used to infect the abdominal cavity of mice to construct a sepsis mouse model; the sepsis mice were divided into 3 different doses (5mg / kg , 10mg / kg and 20mg / kg) lisudil treatment group and negative control group, after 5 minutes of E.coli infection in mouse abdominal cavity, give 5mg / kg, 5mg / kg, 10mg / kg and 20mg / kg of risudil, and the negative control group mice were given the same dose of solvent (distilled water) intraperitoneally; choose healthy mice at the same time, set the blank control group and risudil control group, wherein, Lisudil 20 mg / kg was administered intraperitoneally to the mice in the lisudil control group, and the same dose of solvent (distilled water) was administered to the mice in the blank control group. Observe the 72h survival rate of each group of mice, the observation results are shown in figure 1 .
[0055] Depend on figure 1 It can be seen that the 7...
Embodiment 2
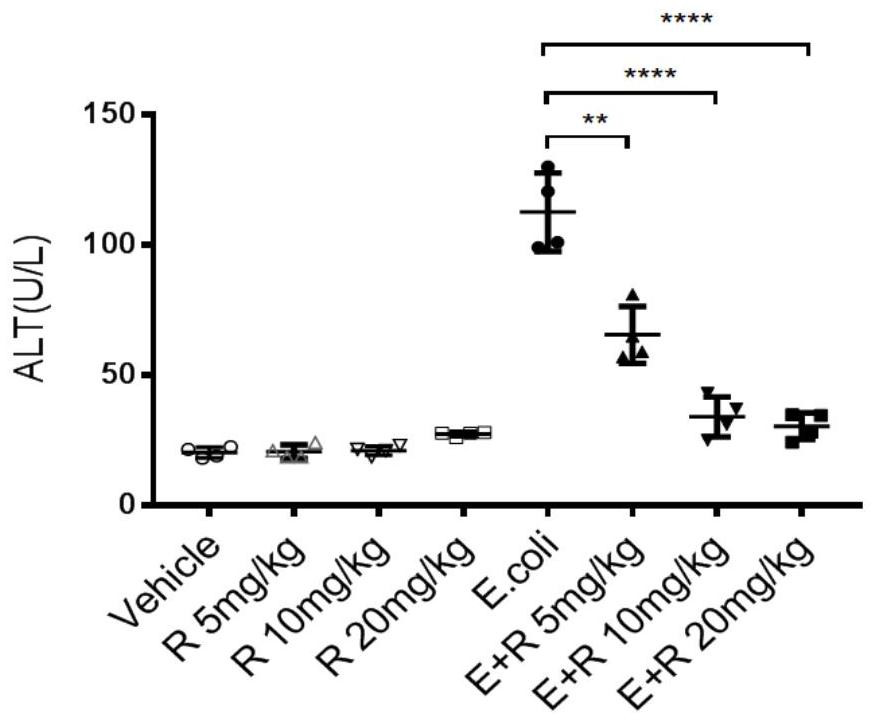
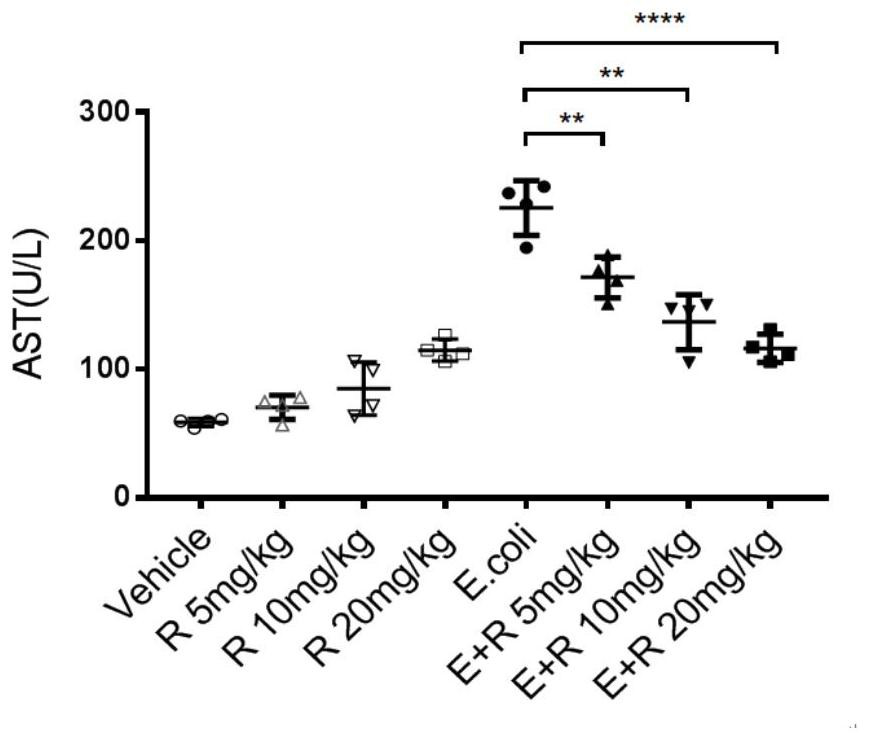
[0057] Adopt the same method as Example 1 to construct the sepsis mouse model, and set the same Lisudil treatment group (5mg / kg, 10mg / kg and 20mg / kg), negative control group and blank control group as in Example 1 group and lisudil control group (5mg / kg, 10mg / kg and 20mg / kg) mice, each group of four mice; after detection of abdominal cavity E.coli infection 12h, alanine aminotransferase (ALT ), aspartate aminotransferase (AST), creatinine (CREA), blood urea nitrogen (BUN) concentration levels, the test results are shown in sequence figure 2 , image 3 , Figure 4 and Figure 5 .
[0058] Depend on figure 2 , image 3 , Figure 4 and Figure 5 It can be seen that the ALT level, AST level, CREA level and BUN level of the mice in each lisudil control group are equivalent to those of the blank control group, indicating that lisudil has no toxic effect on mice and does not cause liver and kidney damage.
[0059] Depend on figure 2 It can be seen that after 12 hours of i...
Embodiment 3
[0069] Adopt the same method as Example 1 to construct the sepsis mouse model, and set the same Lisudil treatment group (20mg / kg), negative control group, blank control group and Lisudil control group as Example 1 (Mice, after detection of E.coli infection in the abdominal cavity for 12 hours, the bacterial load in the blood and peritoneal lavage fluid of each group of mice, and on the liver, lung, spleen and kidney, the test results are shown in Table 2, Figure 10 , Figure 11 , Figure 12 , Figure 13 , Figure 14 and Figure 15 shown.
[0070] Table 2
[0071]
[0072] From Table 2, Figure 10 , Figure 11 , Figure 12 , Figure 13 , Figure 14 and Figure 15 It can be seen that the administration of lisudil after E.coli infection in the abdominal cavity can significantly reduce the bacterial load in the blood, abdominal cavity and various organs of septic mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com