Preparation method of pH-responsive organic-inorganic composite bone cement
An inorganic composite, bone cement technology, applied in medical science, tissue regeneration, prosthesis, etc., can solve the problem of pH-responsive drug release of expandable bone cement, to prevent drug resistance, good application prospects, and achieve The effect of smart release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
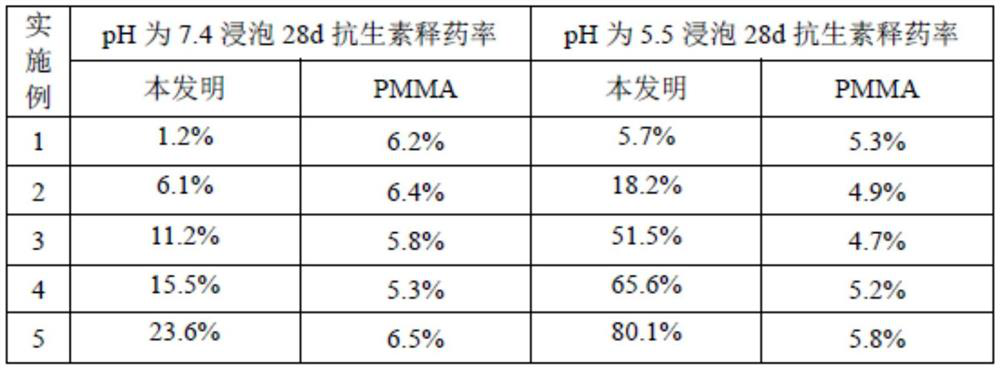
Examples
Embodiment 1
[0038] Dissolve calcium chloride, potassium carbonate and gentamycin in deionized water respectively, stir evenly, pour the gentamicin solution and calcium chloride solution into the potassium carbonate solution, stir with magnetic force to react, and centrifuge the obtained product Obtain drug-loaded calcium carbonate nanoparticles; the mol ratio of calcium chloride to potassium carbonate is 0.8:1; the ratio of gentamicin to the total mass of calcium chloride and potassium carbonate is 1:40; the stirring speed is 800 rpm ; The reaction time is 10min. Disperse drug-loaded calcium carbonate nanoparticles in deionized water and ultrasonically homogenize drug-loaded calcium carbonate suspension; dissolve N,N'-methylenebisacrylamide in deionized water; dissolve azobisisobutyronitrile in In water and ethanol; add drug-loaded calcium carbonate suspension, dimethylaminoethyl methacrylate, N,N'-methylenebisacrylamide aqueous solution and azobisisobutyronitrile ethanol solution in the ...
Embodiment 2
[0040] Dissolve calcium chloride, potassium carbonate and neomycin in deionized water respectively, stir evenly, pour neomycin solution and calcium chloride solution into potassium carbonate solution, magnetically stir for reaction, and centrifuge the obtained product to obtain Drug calcium carbonate nanoparticles; the molar ratio of calcium chloride to potassium carbonate is 0.9:1; the ratio of neomycin to the total mass of calcium chloride and potassium carbonate is 1:45; the stirring speed is 1000 rpm; the reaction time for 20min. Disperse drug-loaded calcium carbonate nanoparticles in deionized water and ultrasonically homogenize drug-loaded calcium carbonate suspension; dissolve N,N'-methylenebisacrylamide in deionized water; dissolve azobisisobutyronitrile in In water and ethanol; add drug-loaded calcium carbonate suspension, dimethylaminoethyl methacrylate, N,N'-methylenebisacrylamide aqueous solution and azobisisobutyronitrile ethanol solution in the reaction vessel, 6...
Embodiment 3
[0042] Dissolve calcium chloride, potassium carbonate and vancomycin in deionized water respectively, stir evenly, pour vancomycin solution and calcium chloride solution into potassium carbonate solution, and react with magnetic stirring, and centrifuge the obtained product to obtain Drug calcium carbonate nanoparticles; the molar ratio of calcium chloride to potassium carbonate is 1:1; the ratio of vancomycin to the total mass of calcium chloride and potassium carbonate is 1:50; the stirring speed is 1200 rpm; the reaction time for 30min. Disperse drug-loaded calcium carbonate nanoparticles in deionized water and ultrasonically homogenize drug-loaded calcium carbonate suspension; dissolve N,N'-methylenebisacrylamide in deionized water; dissolve azobisisobutyronitrile in In water and ethanol; Add drug-loaded calcium carbonate suspension, dimethylaminoethyl methacrylate, N,N'-methylenebisacrylamide aqueous solution and azobisisobutyronitrile ethanol solution in the reaction ves...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com