Novel chiral pyridine-containing polydentate nitrogen ligand and palladium complex as well as preparation method and application thereof
A technology of palladium complexes and nitrogen ligands is applied in the field of novel chiral pyridine-containing polydentate nitrogen ligands, which can solve the problems of low enantiomeric selectivity of products, achieve good enantiomeric selectivity, improve efficiency and yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
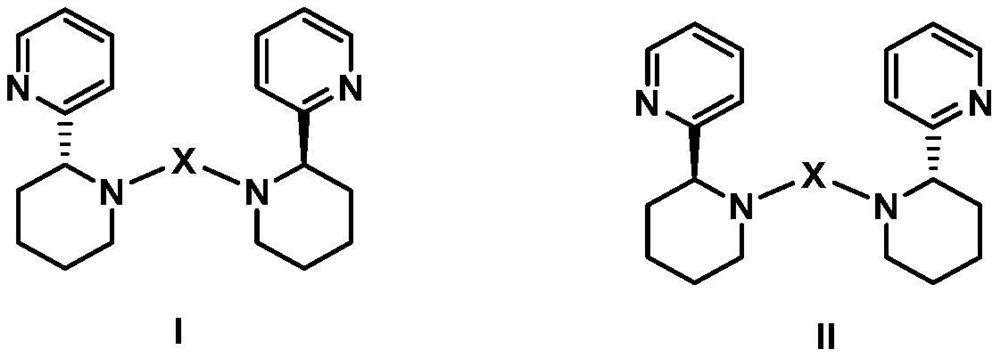
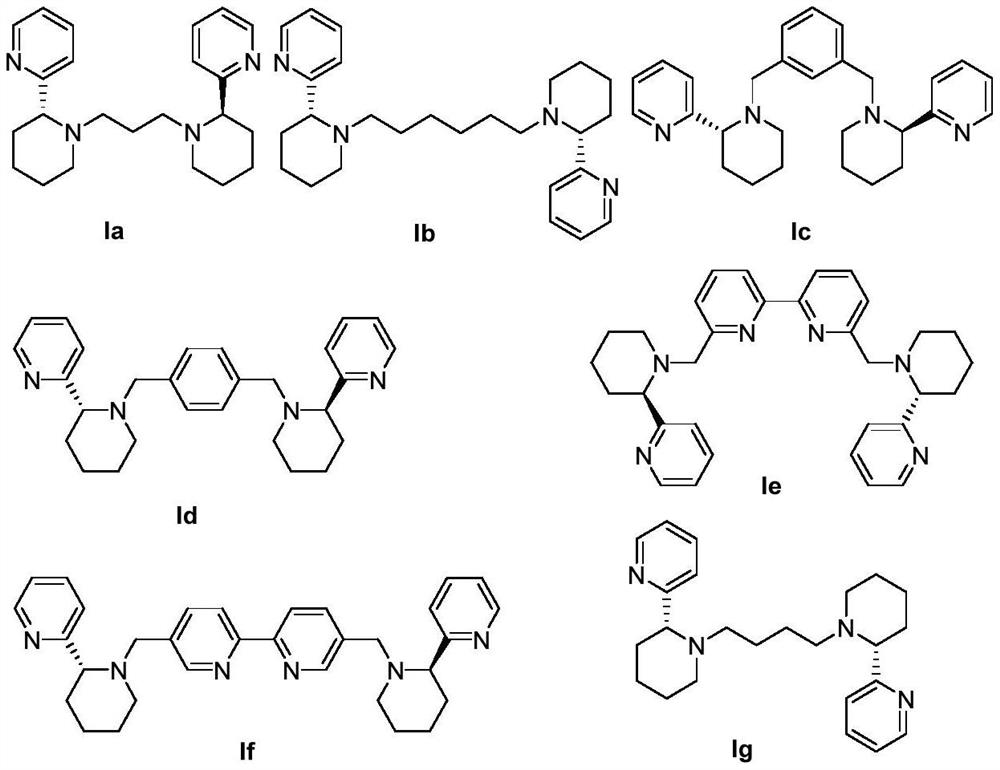
[0033] Example 1 Preparation of novel chiral pyridine-containing multidentate nitrogen ligand Ia
[0034] Dissolve 1mmol of (R)-2-(2-piperidinyl)pyridine in acetonitrile to a concentration of 0.3mol / L, dissolve 0.5mmol of 1,3-dibromopropane in acetonitrile to a concentration of 0.15mol / L, and then add 2mmol Anhydrous potassium carbonate, stirred at 70°C until the thin layer chromatography detected that the raw material point disappeared, then cooled, filtered to remove insoluble matter, and the filtrate was evaporated to dryness under reduced pressure, and the evaporated matter was fully dissolved in dichloromethane, and then hydrated with saturated saline three times Fully wash, retain the washed brine, and back-extract with dichloromethane, collect all the dichloromethane used for back-extraction, dry with anhydrous sodium sulfate, filter to get the filtrate, and after rotary evaporation to dryness, purify with silica gel column chromatography. That is, the novel chiral pyri...
Embodiment 2
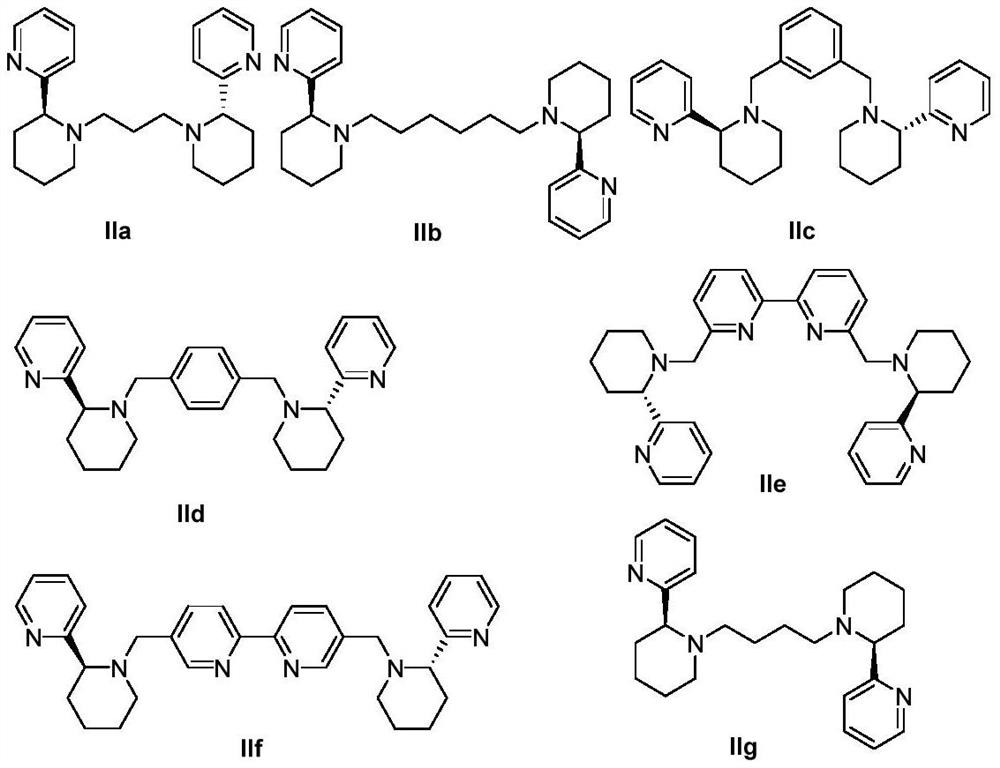
[0038] Example 2 Preparation of novel chiral pyridine-containing polydentate nitrogen ligand IIa
[0039] With the method of Example 1, the difference is that (S)-2-(2-piperidinyl)pyridine is used to replace (R)-2-(2-piperidinyl)pyridine, and finally the novel chiral pyridine-containing polyol is obtained. Dental nitrogen ligand IIa, the structure is as follows:
[0040]
[0041] The NMR spectrogram data are as follows:
[0042] 1 H NMR (CDCl 3 ,300MHz):δ8.47(m,2H),7.55(dt,J=7.8,1.8Hz,2H),7.28(d,J=6.9Hz,2H),7.09(m,2H),3.15(dd, J=10.8,2.1Hz,2H),3.03(d,J=11.4Hz,2H),2.01(m,6H),1.64(m,12H),1.34(m,2H). 13 C NMR (CDCl 3 ,600MHz):δ164.47,148.80,136.34,121.67,70.21,53.64,52.61,34.97,25.84,24.62,22.00.ESI-MS(m / z):[M+H] + 365.2.
Embodiment 3
[0043] Example 3 Preparation of novel chiral pyridine-containing polydentate nitrogen ligand Ib
[0044]Same as the scheme in Example 1, the difference is that 1,3-dibromopropane is replaced by 1,6-dibromohexane, and the stirring temperature is 60°C to finally obtain the novel chiral pyridine-containing polydentate nitrogen ligand Ib, The structure is as follows:
[0045]
[0046] The NMR spectrogram data are as follows:
[0047] 1 H NMR (CDCl 3 ,400MHz):δ8.50(d,J=4.0Hz,2H),7.60(dt,J=8.0,4.0Hz,2H),7.36(d,J=8.0Hz,2H),7.11(m,2H) ,3.19(dd,J=12.0Hz,2H),3.13(d,J=12.0Hz,2H),2.22(m,2H),2.03(m,2H),1.89(m,2H),1.78(d, J=12.0Hz, 4H), 1.67(d, J=12.0Hz, 4H), 1.52(m, 2H), 1.34(m, 6H), 0.96(m, 4H). 13 C NMR (CDCl 3 ,600MHz):δ164.82,148.88,136.36,121.73,121.62,70.52,55.76,52.94,35.14,27.22,26.03,25.92,24.72.ESI-MS(m / z):[M+H] + 407.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com