Preparation method of progesterone vaginal gel
A technology of vaginal gel and progesterone, which is applied in the field of drug preparation, can solve the problems of difficulty in controlling the content uniformity of raw materials, rapid swelling, etc., and achieve the effect of solving rapid polymer swelling, simple operation process, and convenient large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Prepare raw materials according to prescription,
[0093] Prepare as follows:
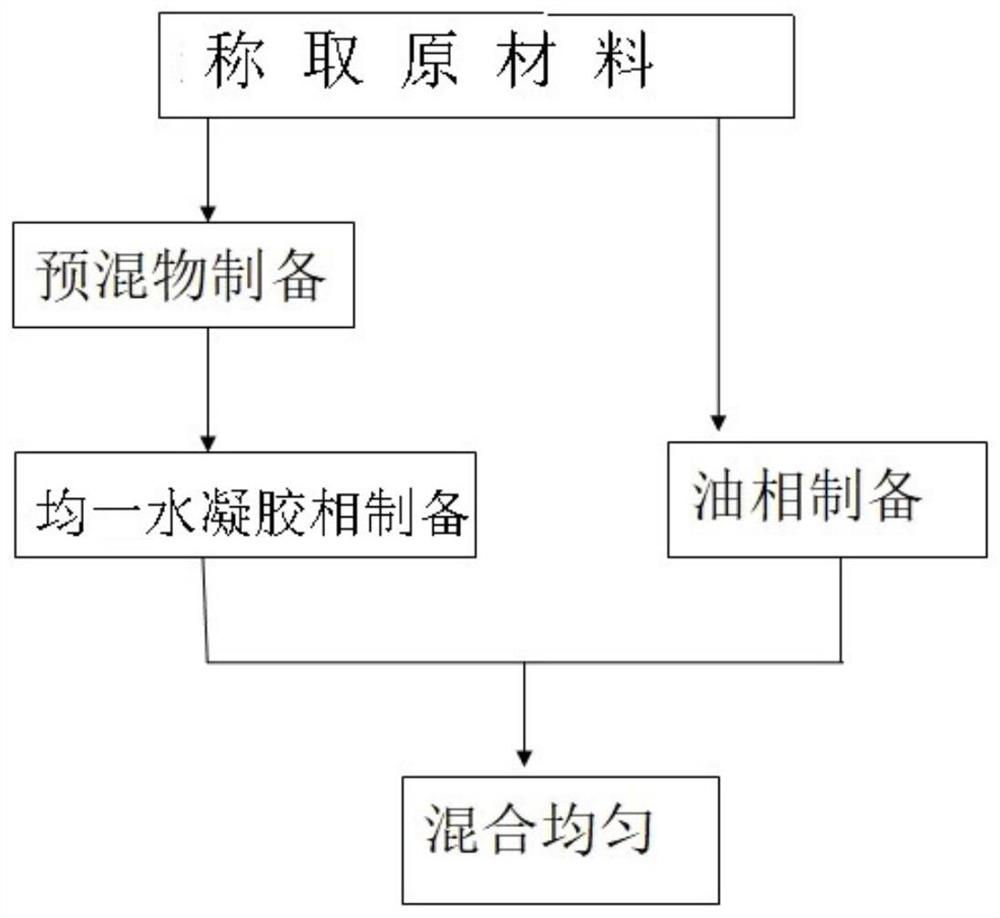
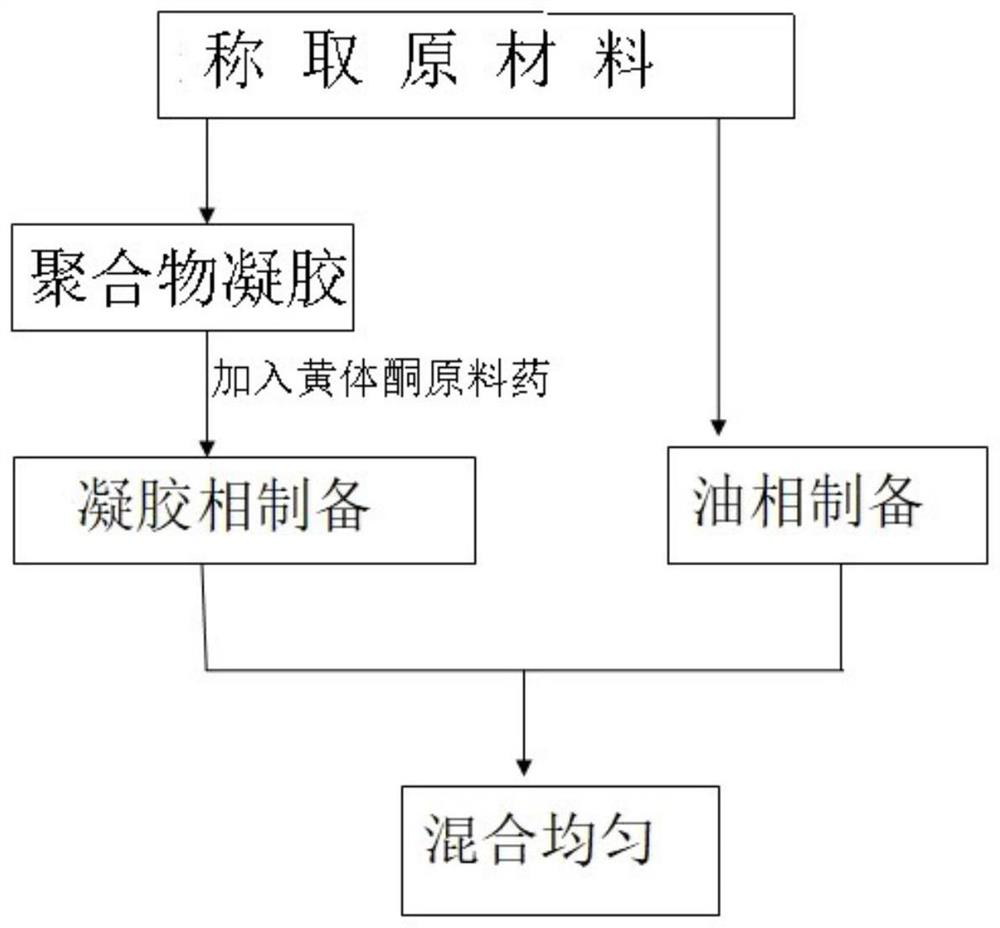
[0094] a, the preparation of the premix of raw material drug and polymer, mix progesterone, carbomer, and polycarbophil in the mixing tank according to the prescription ratio, mix at a speed of 10 rpm, and take 6 minutes to obtain the premix,
[0095] b. Water phase preparation, add glycerin and sorbic acid into purified water according to the prescription ratio, stir to dissolve, stirring at a speed of 10 rpm for 1 hour;
[0096] c. Add the premix into the water phase under stirring conditions, heat and stir until a uniform gel phase is formed, stir rapidly at 1500 rpm, stir slowly at 25 rpm, and stir for 2.5 hours to obtain a hydrogel phase;
[0097] d. Preparation of oil phase, heating liquid paraffin and hydrogenated palm oil to 75°C to melt, and mixing evenly;
[0098] e. Heat the hydrogel phase to the same temperature as the oil phase, mix the two phases, stir rapidly at 1500rpm, sti...
Embodiment 2
[0109] Prepare raw materials according to prescription,
[0110] Prepare as follows:
[0111] Others are the same as in Example 1, except that: in step a, the mixing speed is 30 rpm, and the time is 5 minutes; in step d, it is heated to 55° C. to melt.
[0112] The evaluation process is the same as in Example 1.
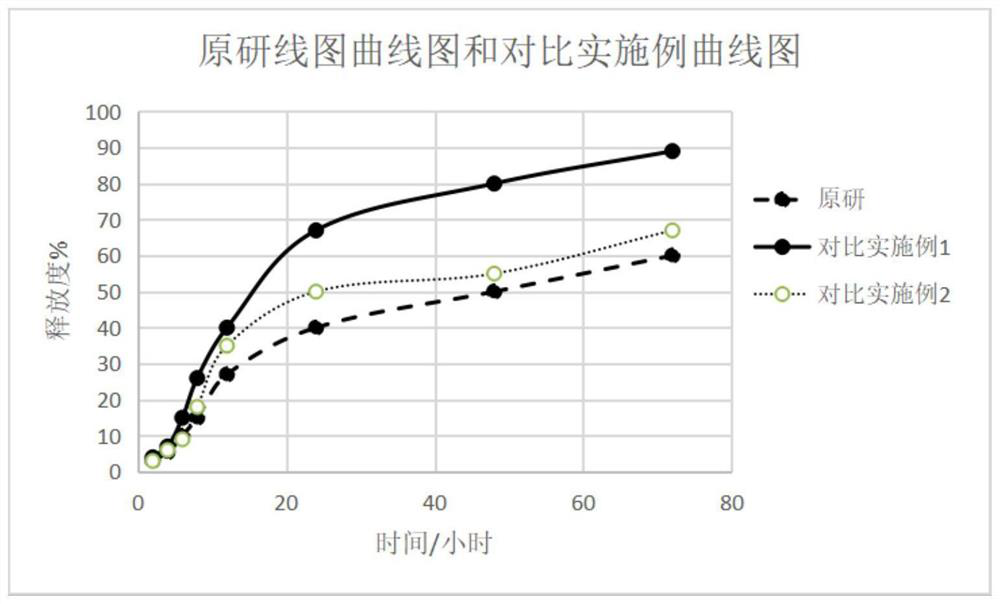
[0113] The evaluation results of appearance and mixing uniformity are shown in Table 3. The release results are as Figure 5 shown.
Embodiment 3
[0115] Prepare raw materials according to prescription,
[0116] Prepare as follows:
[0117] Others are the same as in Example 1, except that: in step a, the mixing speed is 25 rpm, and the time is 10 minutes; in step c, the stirring speed is 1000 rpm.
[0118] The evaluation process is the same as in Example 1.
[0119] The evaluation results of appearance and mixing uniformity are shown in Table 3. The release results are as Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com