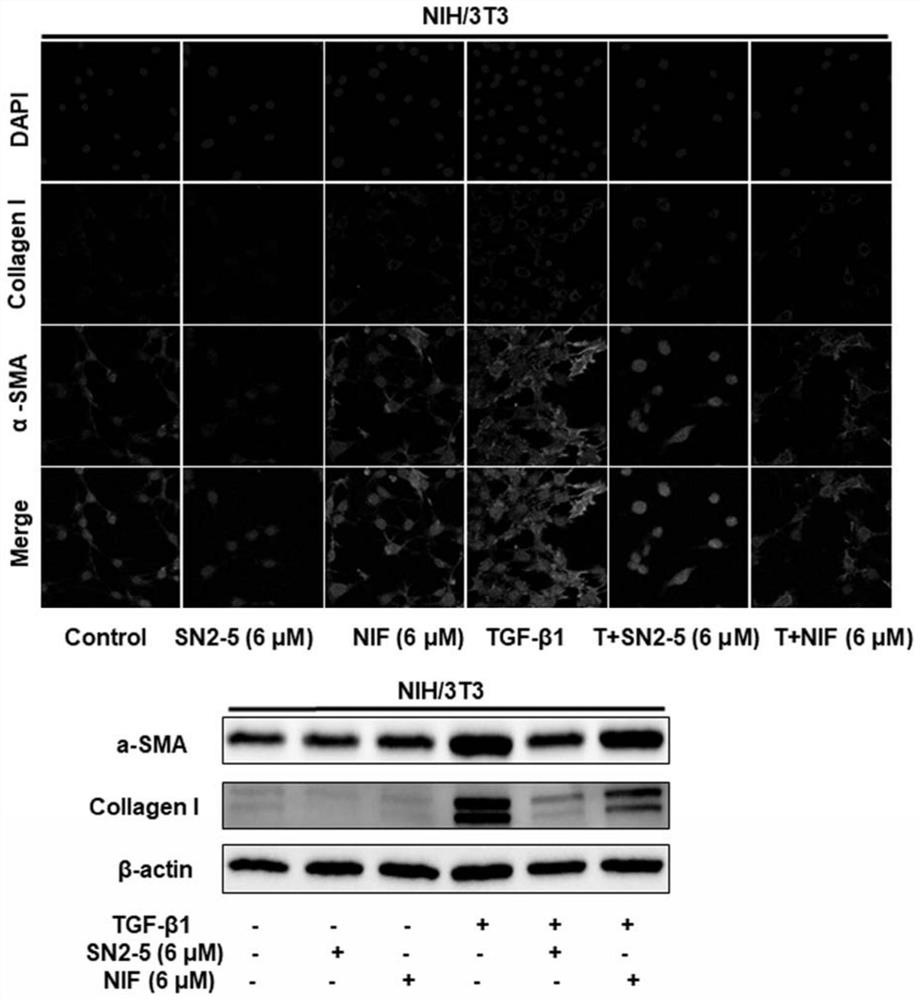
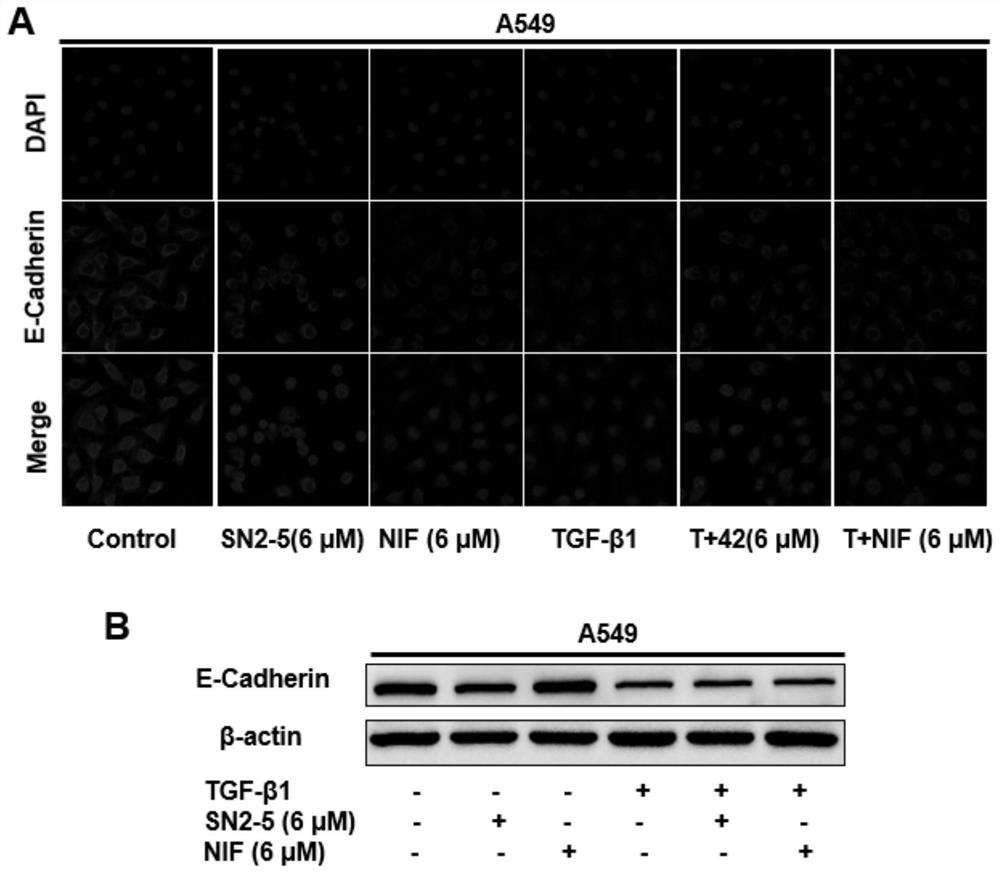
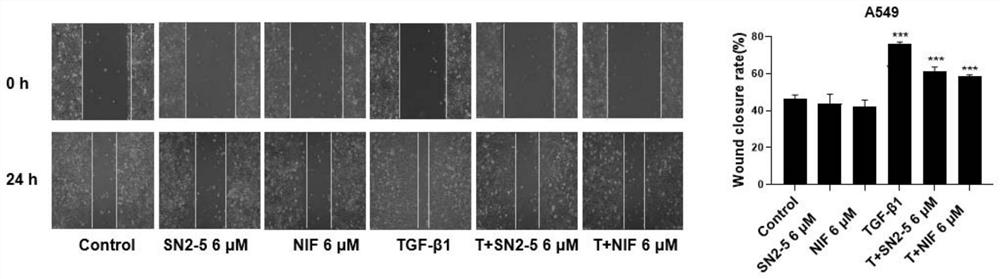
Nifurazide derivatives and their preparation methods and uses
A technology of drugs and compounds, applied in the field of chemical medicine, can solve the problem that pulmonary fibrosis needs to be further improved, and achieve the effects of inhibiting epithelial cell-mesenchymal transition, reducing the proliferation of collagen fibers in lung tissue, and having less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Preparation of compound SN2-5
[0093]
[0094] Take raw material 1 (10 mmol), cesium carbonate (15 mmol), dissolve in DMF (15 mL), add compound 2 (50 mmol) and react at 60 ° C for 1 h. After TLC detects the reaction is complete, use ethyl acetate / water / saturated common salt successively After extraction with water, the organic phase was concentrated to obtain a crude product, which was purified by column chromatography (eluent: PE / EA=20 / 1-8 / 1) to obtain Intermediate 1. White solid, 90% yield.
[0095] Wherein, PE is petroleum ether, and EA is ethyl acetate.
[0096]
[0097] The intermediate 1 (1 mmol) and potassium carbonate (2 mmol) were dissolved in acetonitrile (10 mL), piperidine (2 mmol) was added, and the reaction was refluxed at 85° C. for 3 h. After the completion of the reaction was detected by TLC, the solvent was distilled off under reduced pressure, followed by extraction with ethyl acetate / water / saturated brine, and the organic phase was...
Embodiment 2
[0104] Example 2 Preparation of compound SN1-7
[0105]
[0106] Take nifurazide (1 mmol, 276 mg), KI (potassium iodide, 0.1 mmol, 20 mg) and dissolve in DMF (10 mL), then add 1,3-dibromopropane (1.4 mmol, 143 μL) and DIEA (N , N-diisopropylethylamine, 1.5 mmol, 0.5 mL) was reacted at 80 ° C for 5 hours, TLC detection, after the reaction was complete, the reaction system was added to 100 mL of water, extracted with ethyl acetate 3 times, and then with saturated salt After extraction with water once, the combined organic phases were concentrated to obtain the crude product, which was purified by thin layer chromatography (eluent: DCM / MeOH=50 / 1) to obtain the final product SN1-7. Yellow solid, 50% yield.
[0107] Referring to the method of Example 2, the raw materials were replaced with corresponding raw materials to prepare compounds SN1-1 to SN1-6 and SN1-8 to SN1-29 of the present invention.
[0108] Table 1 Hydrogen spectrum and mass spectrum data of the compounds of th...
experiment example 1
[0119] Solubility test of compound of experimental example 1
[0120] 1. Experimental method
[0121] The water solubility of the compounds was determined by high performance liquid chromatography. Taking compound SN1-25 as an example, the water solubility test method is as follows: compound SN1-25 is prepared with methanol as solvent at 2, 1, 0.5, 0.25, 0.1, 0 mg / mL, and the standard curve is obtained by HPLC determination. Then compound SN1-25 (2 mg) was added to 1.5 mL EP tubes, and 1 mL of pure water was added. The EP tubes were sonicated for 30 seconds, and allowed to stand for 1 h to obtain a saturated solution. High performance liquid chromatography was used to test the compound SN1- in the saturated solution. 25, that is, the water solubility of compounds SN1-25. The test methods for the water solubility of the remaining compounds were carried out with reference to the SN1-25 compound. Nifurazide was used as a control.
[0122] 2. Experimental results
[0123] Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com