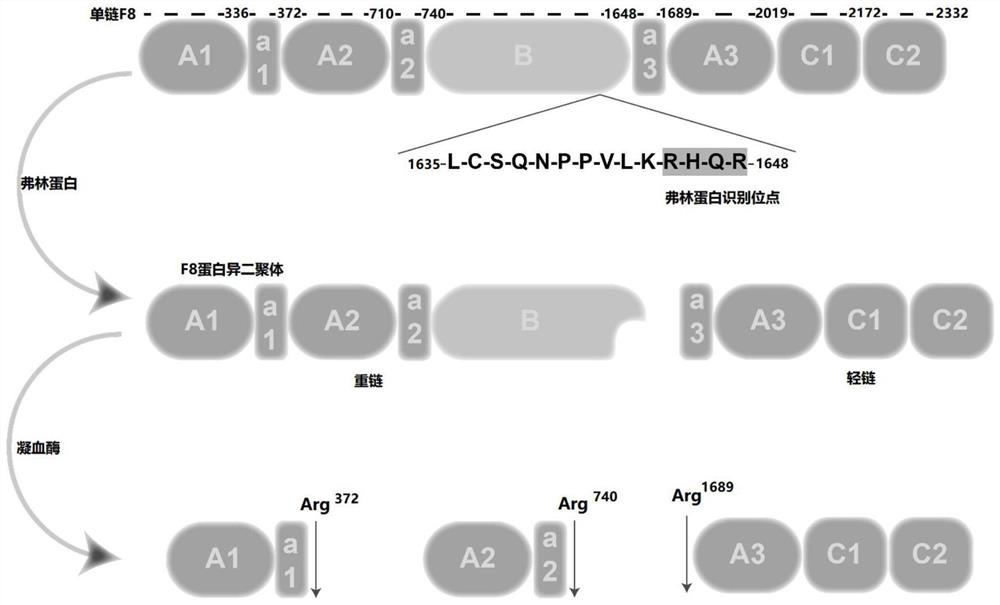
F8 protein variant and gene therapy vector prepared by using same
A protein variant and gene therapy technology, applied in gene therapy, using vectors to introduce foreign genetic material, vectors, etc., to achieve the effects of increasing production titers, reducing the amount of vectors, and improving efficacy and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1 vector construction
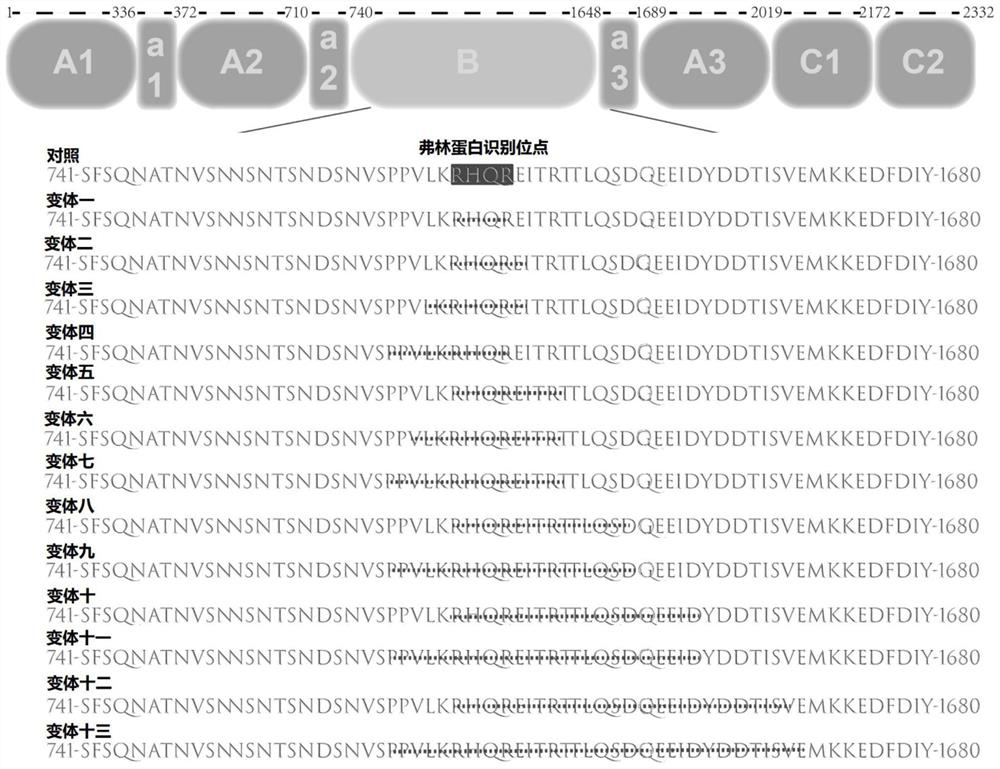
[0072] First, use the KAPAHiFi PCR amplification kit to amplify the BDDF8 sequence; wherein, the BDDF8-N6 nucleic acid sequence (SEQ ID NO.17) is shown; the sequence of the amino acid that is expected to be deleted is directly synthesized into gBlocks at IDT; the plasmid backbone is digested by enzymes and PCR, and then use the NEBuilder HiFi DNA Assembly kit to assemble each fragment.
[0073] All vector sequences were verified using endonuclease and Sanger sequencing to obtain plasmids expressing variants 1 to 13.
Embodiment 2
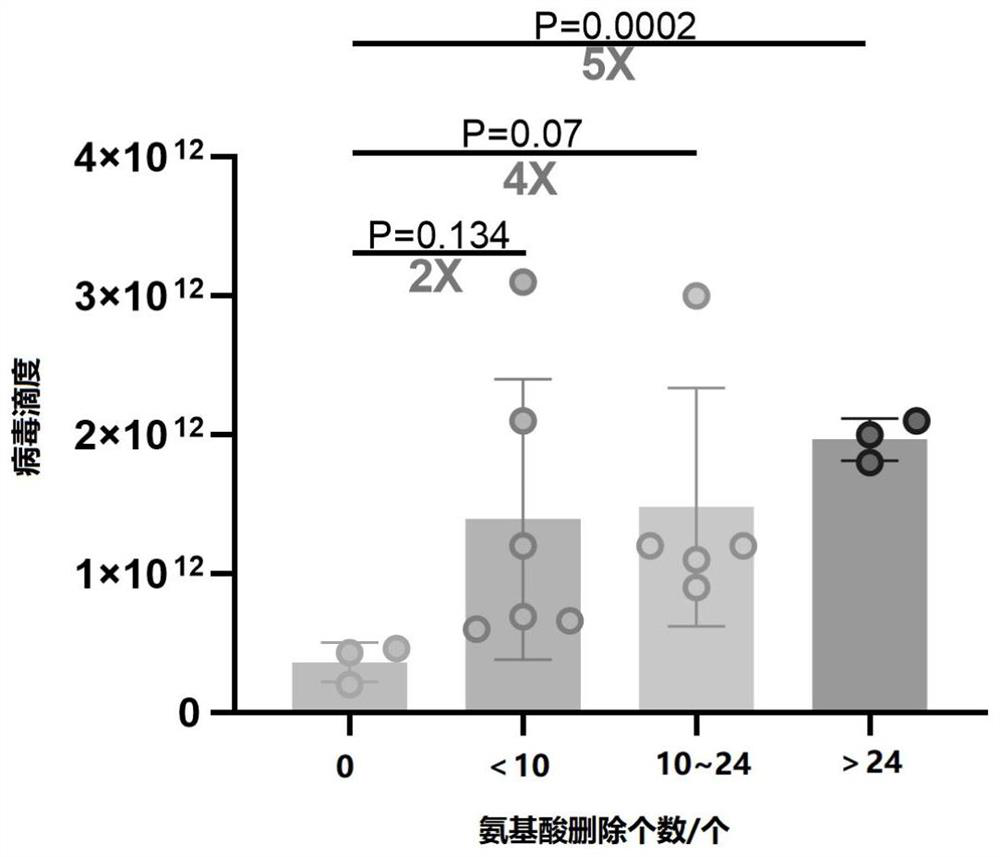
[0074] Example 2 AAV virus packaging, production, concentration, purification and titer determination
[0075] Utilize AAV three-plasmid packaging system for packaging, the three-plasmid packaging system includes: 1) destination vector pAAV-BDDF8-N6-variant; 2) trans plasmid pAAV2 / 8, containing rep and cap genes; 3) adenovirus helper plasmid pHelper (Cell Biolabs), the ratio of the amount of each plasmid is 2:1:1;
[0076] PEI "Max" is used to transfect 293T cells to produce AAV, and the 293T cells are packaged when the confluence of 293T cells is about 80-90%. The base was concentrated about 50 times, and then the AAV vector was purified by iodixanol density gradient centrifugation.
[0077] The AAV titer was determined by qPCR. The iodixanol-purified virus was diluted 3 times with 0.1×TE+20ng / μL SalmonDNA diluent, and heated at 98°C for 5 minutes. Use diluent 0.1×TE+2ng / μL Salmon DNA to dilute the heated AAV virus, and make 5 consecutive 3-fold (5μL+10μL) dilutions.
[00...
Embodiment 3
[0085] Example 3 F8 Activity Detection
[0086] In this example, the AAV8-BDDF8-N6 variant was used to treat hemophilia A mice and followed up to detect F8 activity. Exon 16 of the F8 gene in hemophilia A mice is knocked out, resulting in the abnormal expression of coagulation factor F8, and the F8 activity test results are usually 6% to 8%, resulting in coagulation disorders.
[0087] AAV8-BDDF8-N6 variant injected into hemophilia A mice as Figure 4 As shown, the hemophilia A mice of 6 to 8 weeks were injected to produce the dominant AAV-F8 variant of the present invention, injected uniformly through the tail vein, and the injection dose was 2E+12vg / kg, and the mice were observed every week after injection. The physical condition of the mice was monitored, and blood was collected from the mice at 4, 6, 10, and 20 weeks after injection to detect the activity of F8.
[0088] (1) Blood collection and plasma separation from hemophilia A mice
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com