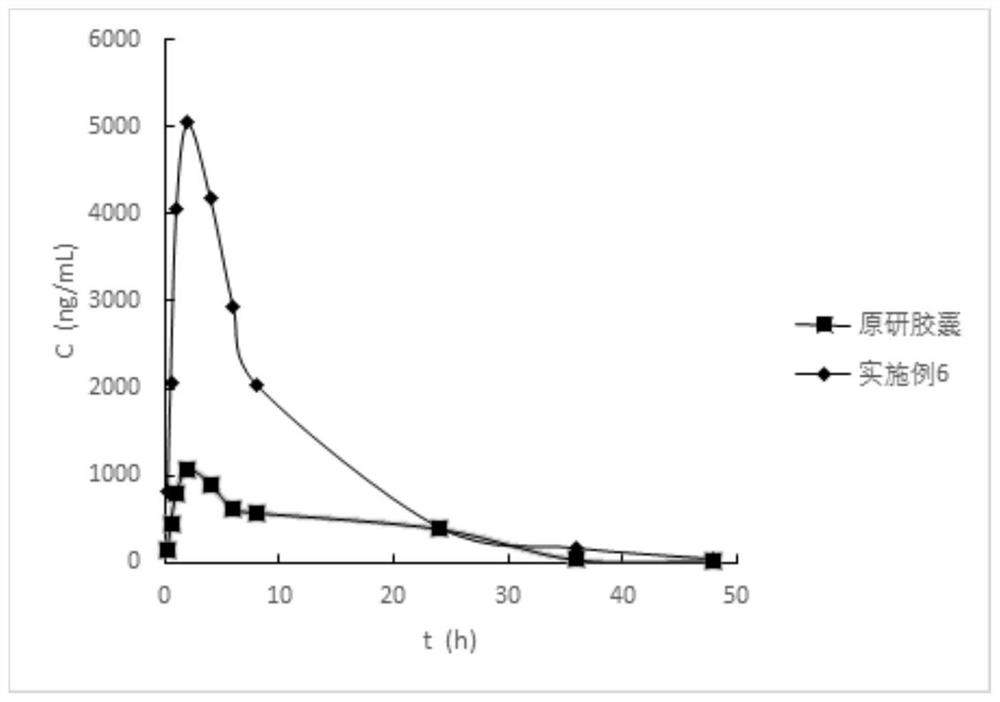
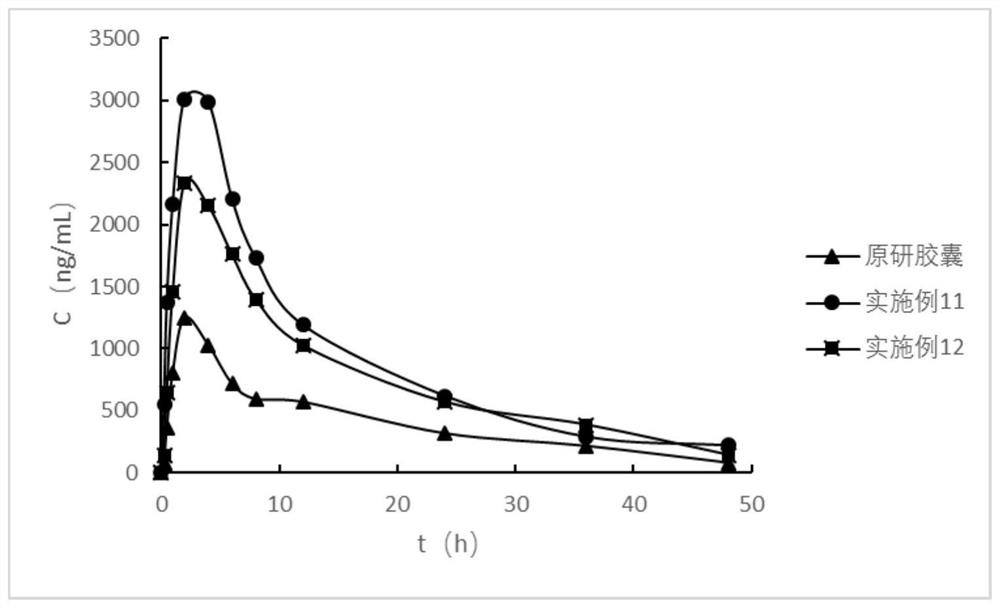
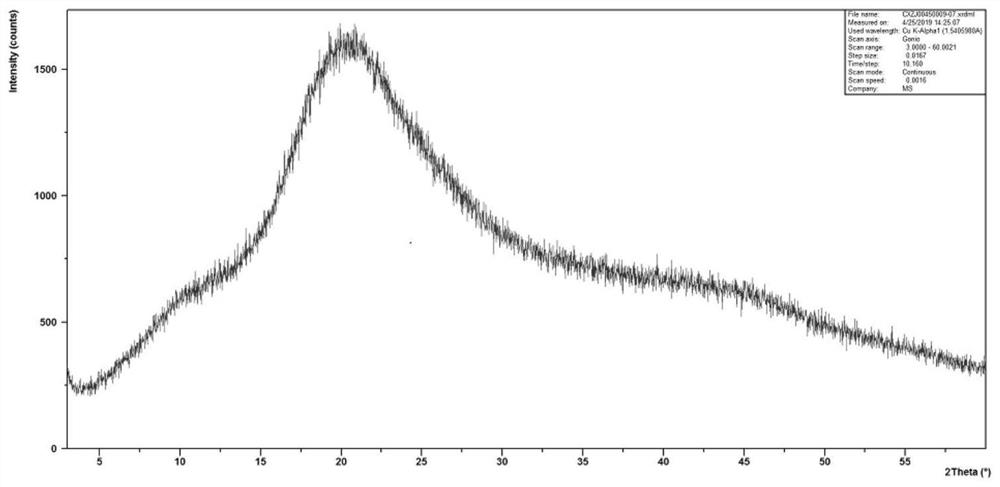
Anti-inflammatory composition and preparation method thereof
A technology of composition and crystallization inhibitor, which is applied in anti-inflammatory agents, drug combinations, pharmaceutical formulas, etc., can solve problems affecting patient compliance, capsules cannot be filled, and tablet weight is too large, so as to improve the stability of the dissolution platform , Improve bioavailability, reduce the effect of carrier usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: HPMCAS single carrier prescription
[0075] Solid dispersion preparation: adopt the hot melt extrusion process to prepare the solid dispersion according to the following formula:
[0076]
[0077] Dissolution results: Take the solid dispersions of the above prescriptions 1-8, place them in dissolution cups containing 1000mL of pH 6.8 buffer salt dissolution medium, rotate at 50rpm, and temperature 37°C, respectively for 10min, 20min, 30min, Sampling 2mL at 60min, and supplementing 2mL of dissolution medium respectively at the same time, the sampled 2mL was filtered through a 0.45μm microporous membrane, and the filtrate was then used to measure the content of celecoxib by high performance liquid chromatography, and calculate the dissolution percentage. The dissolution results are shown in the following table shown.
[0078]
[0079] in conclusion:
[0080] (1) For prescriptions 1-3, when the drug loading ratio is fixed at 1:1, appropriately increas...
Embodiment 2
[0083] Embodiment 2: PVPVA64 single carrier prescription
[0084] Solid dispersion preparation: adopt the hot melt extrusion process to prepare the solid dispersion according to the following formula:
[0085]
[0086] Dissolution result: get the solid dispersion of above-mentioned prescription 9-18, carry out dissolution in the phosphate buffer solution of pH6.8 (dissolution condition is the same as embodiment 1), its dissolution result is shown in the following table.
[0087]
[0088] in conclusion:
[0089] (1) The prescription of PVPVA64 single carrier can achieve better dissolution results only when the drug-loading ratio (1:5) is below and the prescription (prescription 17) with TPGS is added; the high drug-loading ratio (1:1-1 :4), especially the formulations with a drug-loading ratio of 1:1-1:3 have poor dissolution results.
[0090] (2) In PVPVA64 single-vehicle formulations (prescriptions 9-12) with a drug-loading ratio of 1:1-1:2, the addition of TPGS had n...
Embodiment 3
[0091] Embodiment 3: Dissolution and stability results of tablets prepared by prescription 15
[0092] Preparation of tablets: Take the solid dispersion described in prescription 15, microcrystalline cellulose, sodium chloride, croscarmellose sodium and magnesium stearate, and press the following prescription to prepare celecoxib piece:
[0093] Tablet components Prescription ratio (%) Solid dispersion as described in prescription 15 62.50 microcrystalline cellulose 22.00 Sodium chloride 10.00 Croscarmellose Sodium 5.00 Magnesium stearate 0.50 total 100.00
[0094] Stability results: the above tablets were placed in accelerated conditions (40°C, 75%RH) for 1, 2, 3, and 6 months, and placed in long-term conditions (25°C, 60%RH) for 3, 6 months, and samples were taken Carry out content, related substance and dissolution detection (dissolution condition is the same as embodiment 1), and its result is as shown in table 1-table 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com