Method for preparing carbonyl compound by oxidizing alcohol
A carbonyl compound and alcohol oxidation technology, applied in the field of alcohol oxidation to prepare aldehydes or ketones, can solve problems such as water resistance and strong carcinogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
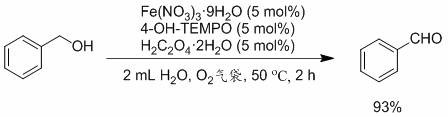
[0016] Embodiment 1: the synthesis of benzaldehyde
[0017]
[0018] Under oxygen atmosphere, Fe(NO 3 ) 3 9H 2 O (60.6 mg, 0.15 mmol), H 2 C 2 o 4 2H 2 O (18.9 mg, 0.15 mmol), 4-OH-TEMPO (25.8 mg, 0.15 mmol), 2 mL H 2 O, benzyl alcohol (324.4 mg, 3 mmol), react at 50 ℃ for 2 h. After the reaction, sodium chloride was added to the mixture for salting out. After filtration, it was extracted with 12 mL of ethyl acetate and dried over anhydrous sodium sulfate. Purified by column chromatography (using a mixed solvent of ethyl acetate / petroleum ether volume ratio of 1:15 as eluent), the isolated yield was 93%. 1 H NMR (400 MHz, CDCl 3 , TMS): δ 10.00 (s, 1H), 7.86 (d, 2H), 7.61 (t, 1H), 7.51 (t, 2H).
[0019] The above 50 ℃ was replaced with room temperature, and the rest remained unchanged. After 2 hours of reaction, the yield was 40%; after 7 hours of reaction, the yield was 91%.
[0020] The above-mentioned oxygen was replaced by air, and the rest remained unchange...
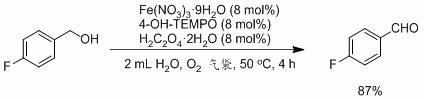
Embodiment 2
[0025] Embodiment 2: the synthesis of p-chlorobenzaldehyde
[0026]
[0027] Under oxygen atmosphere, Fe(NO 3 ) 3 9H 2 O (60.6 mg, 0.15 mmol), H 2 C 2 o 4 2H 2 O (18.9 mg, 0.15 mmol), 4-OH-TEMPO (25.8 mg, 0.15 mmol), 2 mL H 2 O, p-chlorobenzyl alcohol (427.7 mg, 3 mmol), react at 50 ℃ for 4 h. After the reaction, add a small amount of ethyl acetate to the mixture, then add sodium chloride for salting out, filter and extract with 12 mL of ethyl acetate, dry over anhydrous sodium sulfate, and purify by column chromatography (in the form of ethyl acetate / petroleum ether volume The mixed solvent that ratio is 1: 10 is eluent), and yield is 86%. 1 H NMR (400 MHz, CDCl 3 ): δ 9.99 (s, 1H), 7.83 (d, 2H), 7.52 (d, 2H).
[0028] The above-mentioned oxygen was replaced by air, and the rest remained unchanged. The reaction was carried out for 4 h, and the yield was 81%.
Embodiment 3
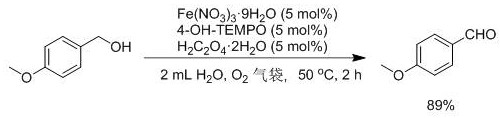
[0029] Embodiment 3: the synthesis of p-methoxybenzaldehyde
[0030]
[0031] Under oxygen atmosphere, Fe(NO 3 ) 3 9H 2 O (60.6 mg, 0.15 mmol), H2 C 2 o 4 2H 2 O (18.9 mg, 0.15 mmol), 4-OH-TEMPO (25.8 mg, 0.15 mmol), 2 mL H 2 O, p-methoxybenzyl alcohol (414.5 mg, 3 mmol), react at 50 ℃ for 2 h. After the reaction, sodium chloride was added to the mixture for salting out. After filtration, it was extracted with 12 mL of ethyl acetate, dried over anhydrous sodium sulfate, and purified by column chromatography (using a mixed solvent of ethyl acetate / petroleum ether volume ratio of 1:10 as eluent), and the yield was 89%. 1 H NMR (400 MHz, CDCl 3 , TMS): δ 9.89 (s, 1 H), 7.84 (d, 2 H), 7.01 (d, 2 H), 3.89 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com