Sulfide electrolyte material and preparation method and application thereof
A technology of sulfide electrolyte and solid electrolyte, which can be used in electrolyte immobilization/gelation, circuits, electrical components, etc., and can solve the problems of poor electrolyte stability, humidity sensitivity, and limited application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] One embodiment of the present invention provides a method for preparing a sulfide electrolyte material, comprising the following steps S10-S20:
[0048] Step S10: Provide raw materials according to the composition and ratio of the above-mentioned sulfide electrolyte materials; the raw materials include Li 6 P.S. 5 X1 or prepare Li 6 P.S. 5 The raw materials needed for X1 and AgX2 and B 2 o 3 ; When y=0, the raw material does not contain B 2 o 3 ;
[0049] Step S20: mixing and sintering the raw materials.
[0050] In some specific examples, in step S10, Li 6 P.S. 5 The raw materials required for X1 include Li X (X=Cl, Br, I), P 2 S 5 and Li 2 S.
[0051] In some specific examples, in step S20, Li X (X=Cl, Br, I), P 2 S 5 , Li 2 S and dopant are mixed and sintered.
[0052] It can be understood that the raw material Li can be provided 6 P.S. 5 X1 (X=Cl, Br, I), or Li X (X=Cl, Br, I), P 2 S 5 and Li 2 S first prepared the sulfur silver germanium ore t...
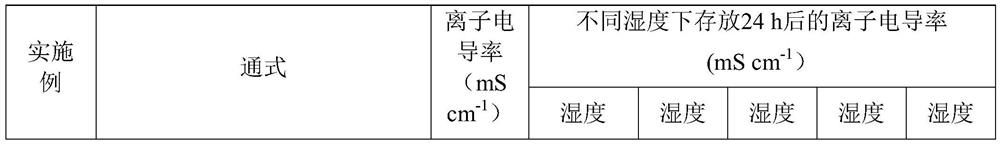
Embodiment 1
[0075] In a glove box filled with high-purity argon, LiCl, P 2 S 5 , Li 2 S according to the sulfur silver germanium type solid electrolyte material Li 6 P.S. 5 The stoichiometric ratio of Cl is weighed, and then according to the molar ratio Li 6 P.S. 5 Cl: Silver chloride = 1:0.1 Weigh the silver chloride, put them together into a ball mill with a rotation speed of 250rpm and mix them for 5 hours, then place them in a muffle furnace at 450°C for sintering for 24 hours. Since the raw material is sintered at 450 °C, the obtained sulfide electrolyte material is denoted as Li 6 P.S. 5 Cl 0.1 AgCl-450.
Embodiment 2
[0077] In a glove box filled with high-purity argon, LiCl, P 2 S 5 , Li 2 S according to the sulfur silver germanium type solid electrolyte material Li 6 P.S. 5 The stoichiometric ratio of Cl is weighed, and then according to the molar ratio Li 6 P.S. 5 Cl: silver chloride: boron oxide = 1:0.05:0.05 Weigh silver chloride and boron oxide, put them together in a ball mill with a rotation speed of 250rpm and mix them for 5h, then place them in a muffle furnace at 450°C for sintering for 24h; The sulfide electrolyte material is denoted as Li 6 P.S. 5 Cl 0.05AgCl 0.05B 2 o 3 -450.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com