Prokaryote-derived Mbp_Argonaute protein and application thereof
A prokaryotic and protein technology, applied in the field of molecular biology, can solve the problems of time-consuming, no targeted cleavage, and high price of chemically synthesized dsRNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
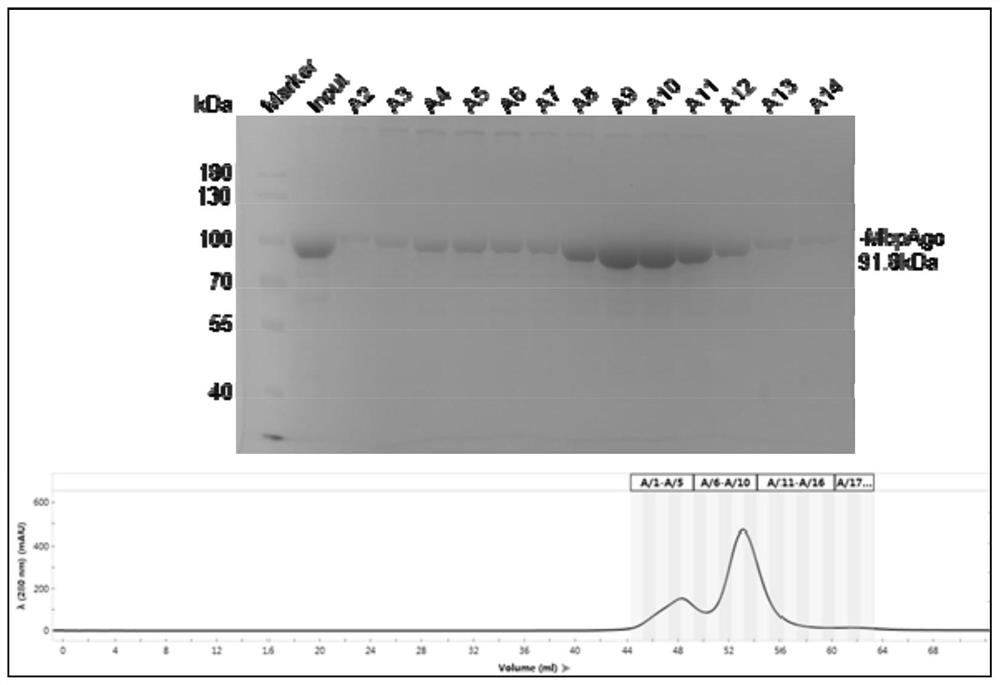
[0052] Example 1 MbpAgo expression and purification
[0053] The nucleotide sequence shown in SEQ ID NO:2 was amplified from the cold-resistant prokaryote Mucilaginibacter paludis, and connected to pET28a by conventional methods to obtain the pET28a-MbpAgo plasmid, and then transformed into Escherichia coli Rosetta (DE3), single colony Inoculate into LB liquid medium containing 50 mg / mL kanamycin, shake the flask on a shaker at 37°C and 220 rpm, when the OD of the bacteria 600 When it reaches 0.8, move to a shaker at 18°C and induce IPTG overnight. Collect the bacteria by centrifugation at 6000rpm for 10min, wash the bacteria with Buffer A (20mM Tris–HCl pH 7.4, 500mM NaCl, 10mM imidazole), resuspend the bacteria in Buffer A, add PMSF at a final concentration of 1mM, and crush under high pressure. Centrifuge at 18000rpm for 30min, and collect the supernatant. After the supernatant was filtered, Ni-NTA purification was performed.
[0054] Wash 10 column volumes each with 2...
Embodiment 2
[0056] Example 2 Determination of MbpAgo cleavage activity
[0057] To assess which combinations of guide RNA / DNA and target RNA / DNA were able to be cleaved by MbpAgo, activity assays were performed for all possible combinations. The sequence diagram of target DNA, target RNA, guide ssDNA and guide ssRNA is as follows Figure 4 , where the arrow indicates the predicted cleavage site
[0058] The cleavage assays were all performed at 37°C with a 4:2:1 (MbpAgo:guide:target) molar ratio. Put 800nM MbpAgo with 400nM guide in the solution containing 10mM HEPES-NaOH, pH 7.5, 100mM NaCl, 5mM MnCl 2 and 5% glycerol in reaction buffer and incubated at 37°C for 10 min for guide loading. Nucleic acid targets were added to 20OnM. After 1 h reaction at 37°C, the reaction was stopped by mixing the samples with 2x RNA loading dye (95% formamide, 18 mM EDTA and 0.025% SDS and 0.025% bromophenol blue) and heating at 95°C for 5 min. The lysate was resolved by 20% denaturing PAGE, stained w...
Embodiment 3
[0060] Example 3 Effect of gDNA length on target RNA cleavage activity
[0061] DNAs with a length of 8-40nt were selected as guide DNAs, incubated with MbpAgo to form pAgo complexes, and the activity of guide DNAs with different lengths to recognize and cut target RNA by MbpAgo was measured. Measurement results such as Figure 7 shown.
[0062] The results show that the length of the guide DNA has a certain influence on the activity of MbpAgo to recognize and cut the target RNA, wherein when the length of the guide DNA is in the range of 8-40nt, preferably 10-30nt, the target RNA can be effectively cut.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com