Continuous synthesis method of ethyl 3-oxo-3-hydrazino-propionate
A technology of ethyl hydrazinopropionate and its synthesis method, which is applied in the field of continuous synthesis of ethyl 3-oxo-3-hydrazinopropionate, and can solve problems such as high energy, large amount of hydrazine hydrate, and poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
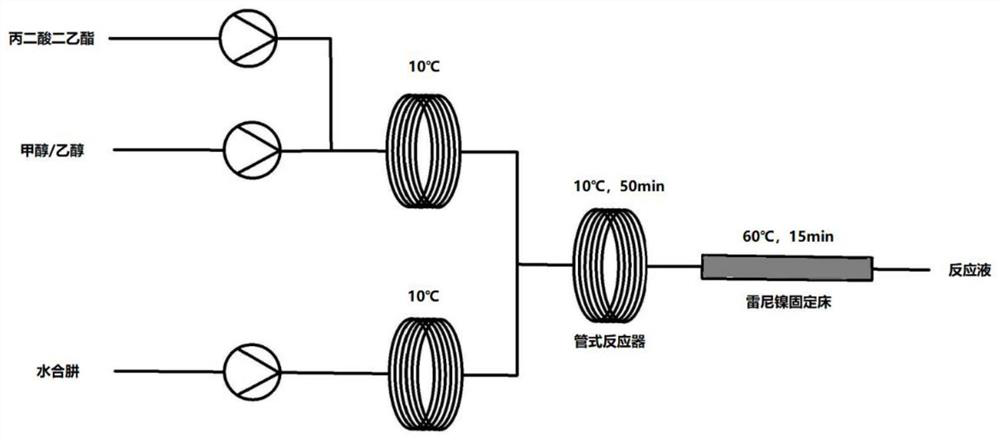
[0040] Such as figure 1 As shown, ethanol and diethyl malonate are pumped to temperature zone 1 (10°C) respectively to form an ethanol solution of raw materials; the flow rate of ethanol is 4mL / min, and the flow rate of diethyl malonate is 1mL / min. Hydrazine hydrate (50wt%) was pumped to temperature zone 2 for pre-cooling to 10°C, with a flow rate of 0.5mL / min. Afterwards, the hydrazine hydrate solution and the ethanol solution of the raw materials are transported into the tubular reactor for hydrazination reaction (temperature zone 3, 10° C.), and the reaction residence time is 50 minutes. The reaction solution flows out into the Raney nickel fixed bed for quenching (temperature zone 4, 60° C.), and the quenching residence time is 15 minutes. After the system reached steady state, samples were collected for 60 minutes. The effluent solution was concentrated, crystallized, and vacuum-dried at 40° C. to obtain 12.50 g of product with a purity of 99.0% and a yield of 21.5%.
Embodiment 2
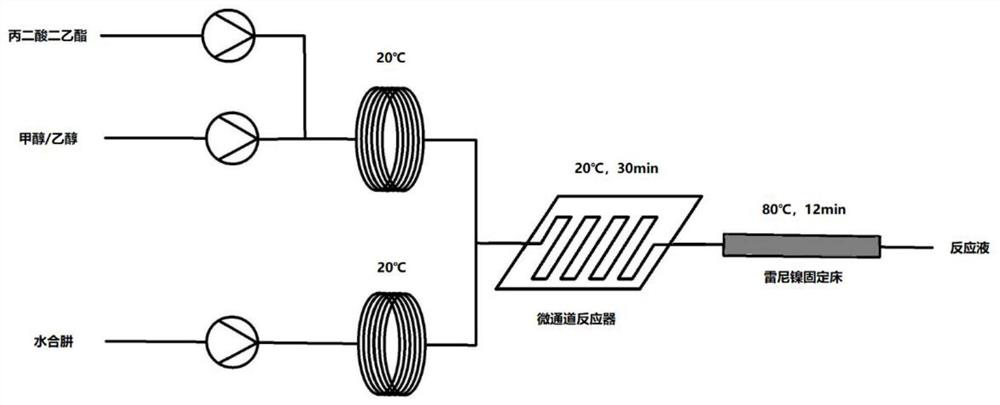
[0042] Such as figure 2 As shown, methanol and diethyl malonate are pumped to temperature zone 1 (20°C) respectively to form a methanol solution of raw materials; the flow rate of methanol is 50mL / min, and the flow rate of diethyl malonate is 50mL / min. Hydrazine hydrate (80wt%) was pumped to temperature zone 2 for pre-cooling to 20°C, with a flow rate of 12mL / min. Afterwards, the hydrazine hydrate solution and the methanol solution of the raw materials are transported into the microchannel reactor for hydrazination reaction (temperature zone 3, 20° C.), and the reaction residence time is 30 minutes. The reaction solution flows out into the Raney nickel fixed bed for quenching (temperature zone 4, 80° C.), and the quenching residence time is 12 minutes. After the system reached steady state, samples were collected for 60 minutes. The effluent solution was concentrated, crystallized, and vacuum-dried at 40°C to obtain 549.5 g of the product with a purity of 98.8% and a yield ...
Embodiment 3
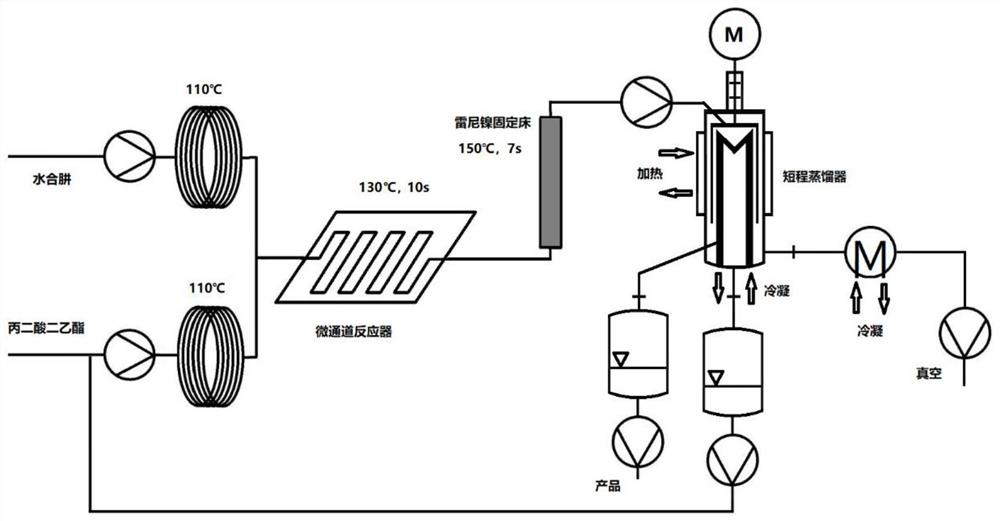
[0044] Such as image 3As shown, diethyl malonate is pumped to temperature zone 1 (110°C), and the flow rate of diethyl malonate is 50mL / min. Hydrazine hydrate (80wt%) was pumped to temperature zone 2 to preheat to 110°C, and its flow rate was 0.8mL / min. Afterwards, the hydrazine hydrate solution and the raw material solution are transported into the microchannel reactor for hydrazination reaction (temperature zone 3, 130° C.), the reaction residence time is 10 s, and the pressure is 12 kG. The reaction solution flows out into the Raney nickel fixed bed for quenching (temperature zone 4, 150°C), and the quenching residence time is 7s; the reaction solution enters the short-path still for separation, and the unreacted diethyl malonate is obtained by the short-path still. Enter Repeat Application. After the system reached steady state, samples were collected for 24 hours. The effluent solution was concentrated, crystallized, and vacuum-dried at 40° C. to obtain 760 g of a pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com