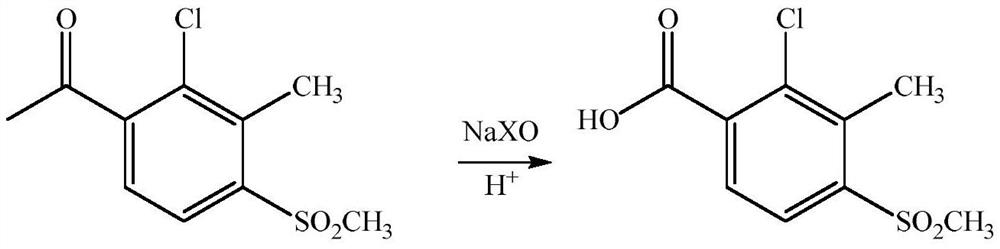
Method for preparing 2-chloro-3-methyl-4-methylsulfonyl benzoic acid
A technology for methanesulfonyl benzoic acid and methanesulfonyl acetophenone is applied in the field of preparing 2-chloro-3-methyl-4-methanesulfonyl benzoic acid, and can solve the problems of complex operation process, high toxicity and easy generation of excessive oxides etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 1mol of 2-chloro-3-methyl-4-methylsulfonylacetophenone, 10mol of 6% sodium hypochlorite aqueous solution (calculated as sodium hypochlorite), and 0.1mol of benzyltriethylammonium chloride are added to the reaction flask, and the temperature is slowly raised to 100°C, keep warm for 0.5h. After the reaction, cool down to 30°C, add 36% hydrochloric acid dropwise to acidify to PH = 1, stir and crystallize for 4 hours, filter, wash the filter cake with 2% aqueous hydrochloric acid, and dry to obtain white powdery solid 2-chloro-3- Methyl-4-methylsulfonylbenzoic acid 247.5g, content 97.9%, yield 97.5%.
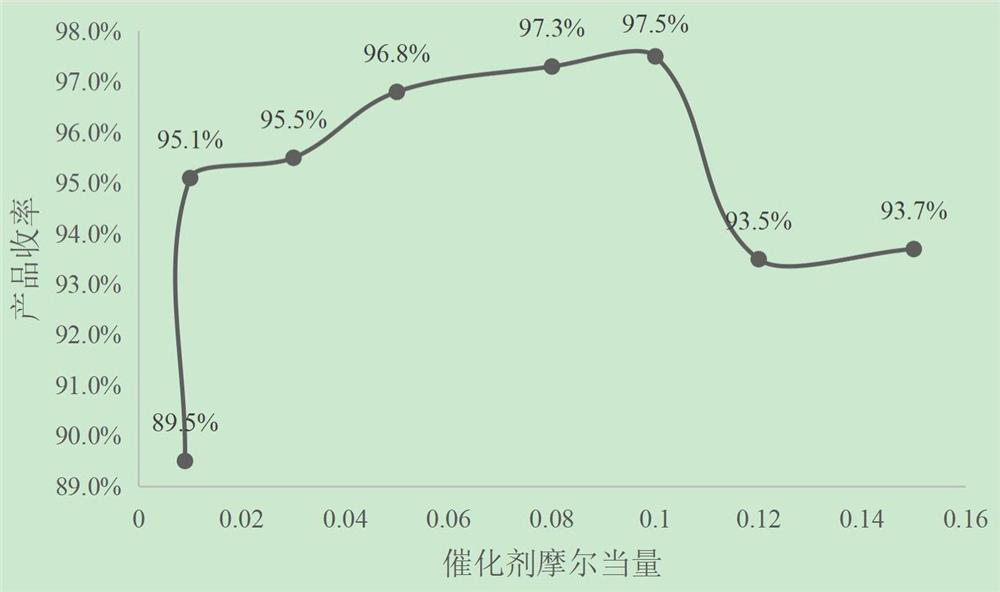
[0028] According to the above reaction conditions and steps, using benzyltriethylammonium chloride as a phase transfer catalyst, the influence of catalysts with different molar equivalents on the reaction results is shown in Table 1.
[0029] The impact of table 1 catalyst dosage on reaction result
[0030] batch number Catalyst molar equivalent Product ...
Embodiment 2
[0033] Weigh 1mol of 2-chloro-3-methyl-4-methylsulfonylacetophenone, 3mol of 15% sodium hypobromite aqueous solution (calculated as sodium hypobromite), and 0.05mol of tetrabutylammonium bromide into the reaction flask , control the temperature to 5°C, and keep the reaction for 8h. After the reaction, cool down to -10°C, add 40% hydrobromic acid dropwise to acidify to PH = 1, stir and crystallize for 0.5h, filter, wash the filter cake with 20% hydrobromic acid aqueous solution, and dry to obtain a white powdery solid 246.2 g of 2-chloro-3-methyl-4-methylsulfonylbenzoic acid, content 97.0%, yield 96.1%.
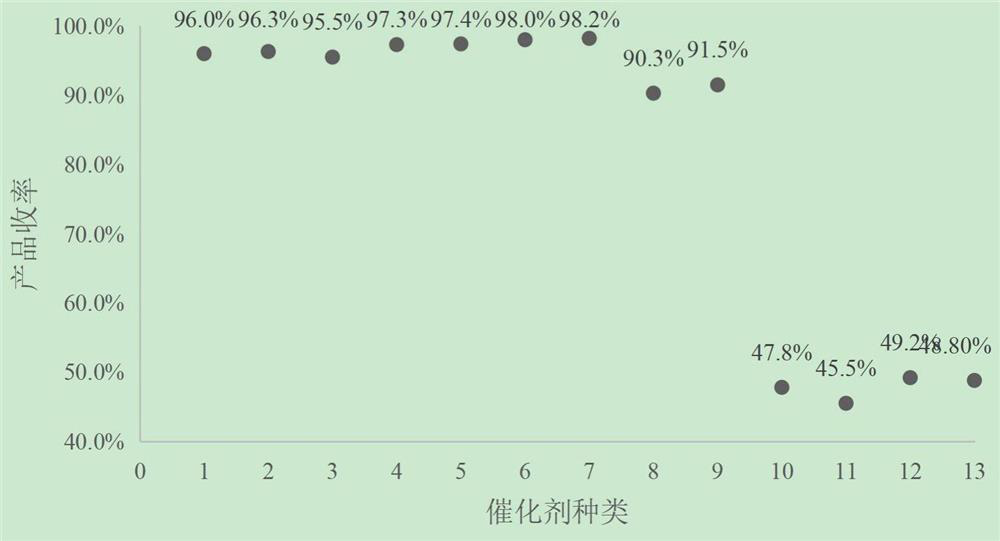
[0034] According to the reaction conditions and steps in Example 2, the impact of different ionic catalyst types on the reaction results is shown in Table 2, and the impact of non-ionic catalyst types on the reaction results is shown in Table 3.
[0035] Table 2 The impact of the type of ionic catalyst on the reaction result
[0036] batch number Types of Ionic C...
Embodiment 3
[0045] Weigh 1 mol of 2-chloro-3-methyl-4-methylsulfonylacetophenone, 5 mol of 10% sodium hypochlorite aqueous solution (calculated as sodium hypochlorite), and 0.01 mol of tetrabutylammonium bisulfate into the reaction flask, and slowly heat up to 55°C , insulation reaction 3h. After the reaction, cool down to 10°C, add 98% sulfuric acid dropwise to acidify to PH = 1, stir and crystallize for 2 hours, filter, wash the filter cake with 10% aqueous sulfuric acid, and dry to obtain white powdery solid 2-chloro-3- Methyl-4-methylsulfonylbenzoic acid 242.1g, content 97.8%, yield 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com