Polypeptide, composition and application thereof
A composition and drug technology, applied in the field of biopharmaceuticals, can solve problems such as inability to exert a good therapeutic effect and the complexity of sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
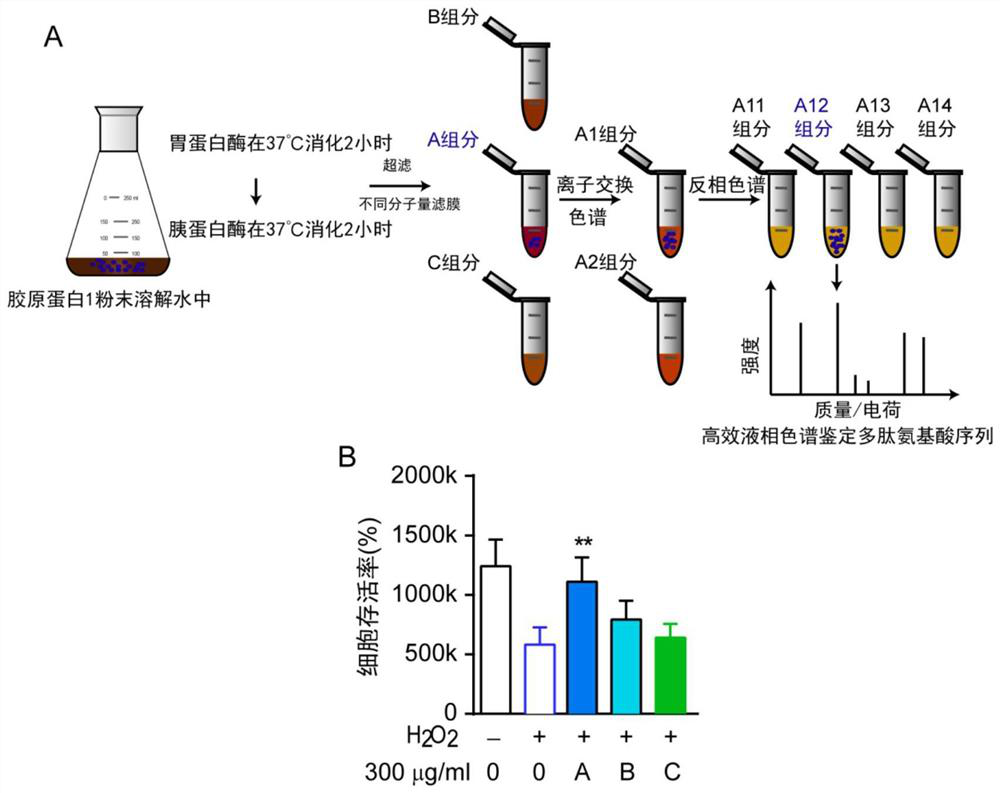
[0100] Example 1 Gradual separation of active ingredients of collagen 1
[0101] (1) First, dissolve donkey collagen 1 (Advanced Biomatrix company product number: 5005) in deionized water at 50 °C to obtain a collagen solution, cool it to 37 °C, and adjust the pH of the collagen solution to 2.0 (It is recommended to use a pH agent for accurate 1 N dilute hydrochloric acid for pH adjustment), add pepsin (Sigma company, product number EC3.4.23.1) according to the weight ratio of pepsin and collagen solution 1:250, at 37 ℃ , digested for 2 hours; then the pH value of the resulting mixture was adjusted to 6.8, and trypsin (Sigma company, product number EC3.4.21.4) was added at a ratio of 1:250 by weight of trypsin and collagen solution. Also at 37 Digest at ℃ for 2h. Subsequently, the collagen solution digested with pepsin and trypsin was heated to 100 °C for 1 hour to inactivate the enzymes. After the solution was naturally cooled, centrifugation was performed at 8000 rpm for 10...
Embodiment 2
[0109] Example 2 The anti-cell death A component is separated by ion exchange chromatography
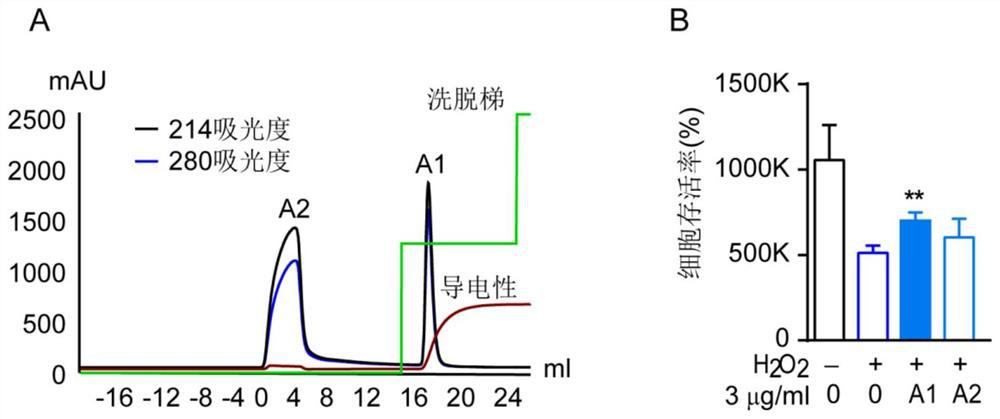
[0110] (1) The A component (molecular weight ≥ 10,000 Da) is separated step by step by ion exchange chromatography. Ion exchange chromatography (IEX) uses IEX technology to purify biomolecules and separate them according to the difference in their net surface charges. This application used AKTA-pure (GE Healthcare, USA) equipped with High-Performance Q (GE healthcare, USA) anion exchange column (100mm*10mm Sepax, USA). Elution was performed with a constant gradient of 50% 1 M NaCl, 50 Mm Tris pH 7.4 at a flow rate of 3 ml / min at room temperature. Absorbance was monitored at 214 and 280 nm. Fractions at the peaks were collected at both A1 and A2 permeabilized and then lyophilized. The lyophilized solution was dissolved in ultrapure water for analysis. For the analysis results, see figure 2 A.
[0111] (2) Detection of the protective effects of the two components A1 and A2 on hyd...
Embodiment 3
[0113] Example 3A1 components were further separated by reversed-phase chromatography
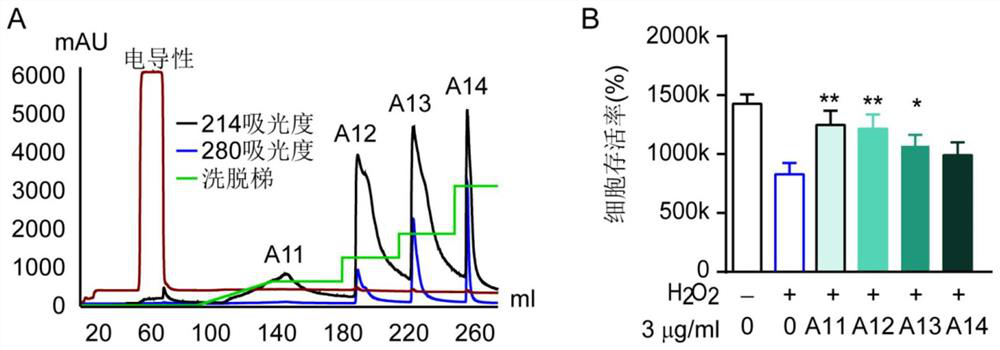
[0114] (1) The A1 component is separated step by step by reversed-phase chromatography, and the RPC separates the fractions according to the hydrophobicity of the substance. The present application used AKTA-pure (GE healthcare, USA) equipped with Source30-RPC (GE healthcare, USA) reversed-phase chromatography column (65mm*10mm Sepax) for fractionation. That is, gradient elution was performed with 0-10% linner first, followed by constant gradient elution with 10 mM Na2HPO4 (pH 7.0) buffer containing 20%, 30%, 50% and 60% acetonitrile, and the flow rate at room temperature was 3ml / min. Finally measure the absorbance at 214 and 280 nm. Peak fractions were collected and lyophilized. The lyophilized solution was dissolved in ultrapure water for analysis. For the analysis results, see image 3 A.
[0115] (2) Detection of the protective effects of four components A11, A12, A13 and A14 on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com