Pharmaceutical composition containing pulsatilla chinensis extract and application thereof
The technology of Pulsatilla and its extract, applied in the field of medicine, can solve problems such as unclear effect of Pulsatilla, and achieve the effects of reducing disease activity index, improving abundance, and repairing intestinal mucosal damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of Pulsatilla Pulsatilla Water Extract (PRW): 250g of Pulsatilla Pulsatillae was purchased from Tongrentang, extracted at 105°C for 1.5 hours in a 1:6 (g / mL) ratio of medicine and water, repeated twice, concentrated and evaporated to the original 1 / 4 of the volume, and then vacuum freeze-dried (-80, -0.06kPa, 48h) for future use.
[0047] Experimental group:
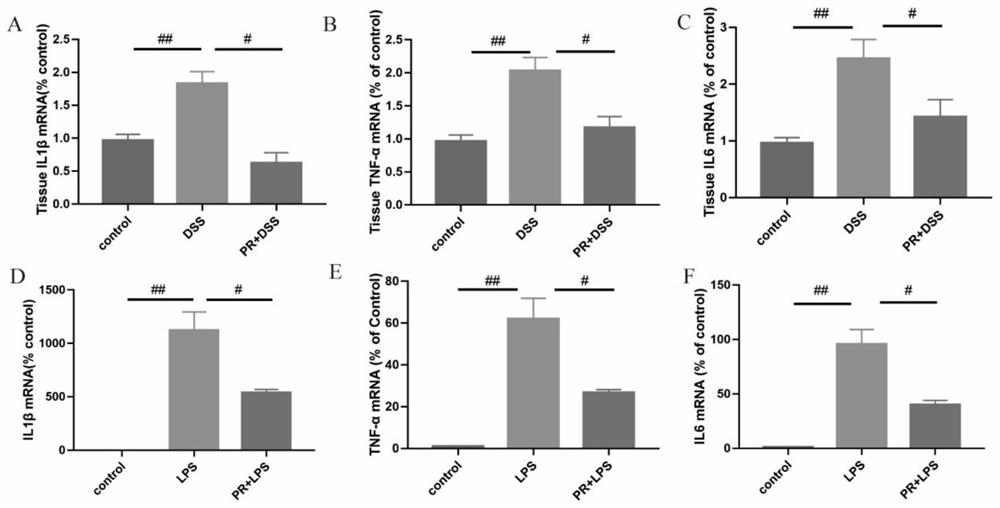
[0048] Female C57BL / 6J mice aged 6-8 weeks were provided by the Animal Center of Shanxi Provincial People's Hospital. The colitis model was established by dextran sulfate sodium salt (DSS) induction. Thirty-six mice were randomly divided into control group, DSS group and PR group. Except for the control group, the mice in the other groups were treated with 3% w / v DSS in drinking water to induce colitis for 7 days.
[0049] Specifically: control group: normal drinking water. DSS group: DSS (40000 kDa; Seebio, Shanghai, China) was added as drinking water at a ratio of 3% w / v. PR group: 3% DSS drinkin...
Embodiment 2
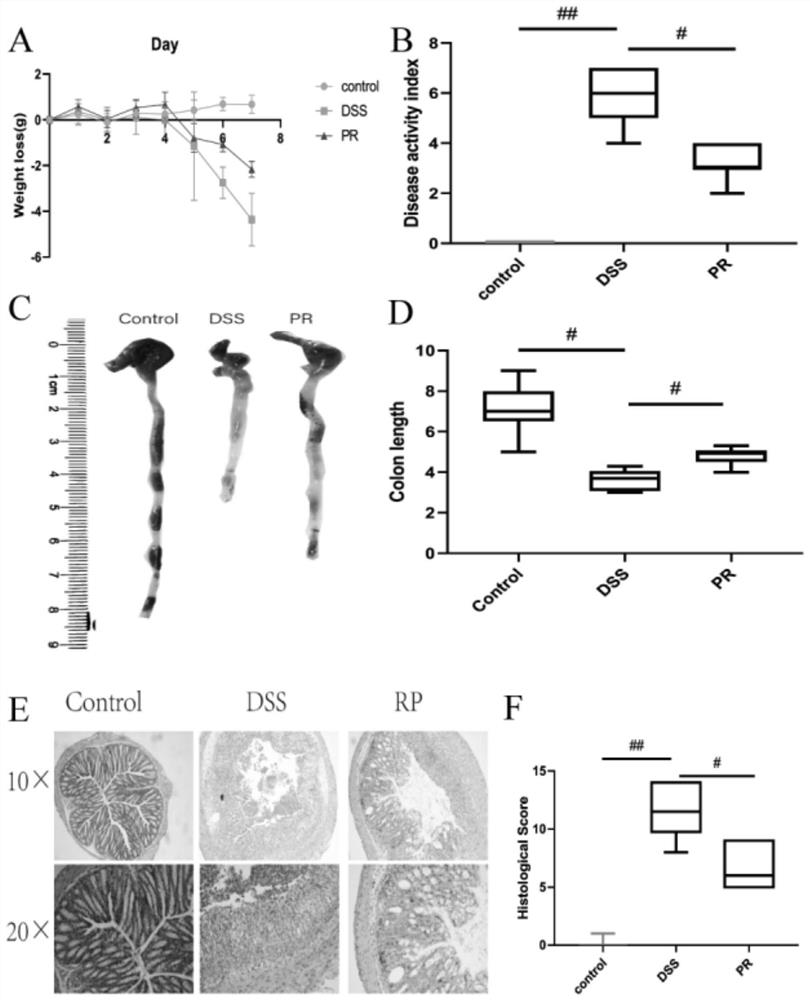
[0051] Embodiment 2 PR is to the improvement of colitis symptom
[0052] Evaluation of colitis: The weight loss, stool consistency and fecal blood content of mice in each group were recorded. Scores for weight loss, stool consistency, and fecal blood content were added to calculate the total disease activity index (DAI). DAI is a clinical parameter reflecting the severity of colitis, and each parameter is scored as follows: weight loss (0: 20%), stool consistency (0: normal; 2: loose stool; 4: diarrhea), blood content in stool (0: occult negative; 2: occult positive; 4: massive bleeding).
[0053] At the end of the experiment, the colon segment was excised, and the length from the ileocecal to the anal verge was measured. Subsequently, formalin (10%) was used for tissue fixation, followed by paraffinization of conical tissue, staining with eosin and hematoxylin (H&E), and blind scoring by an experienced pathologist. Mucus is stained by Meyer mucin.
[0054] The result is as...
Embodiment 3
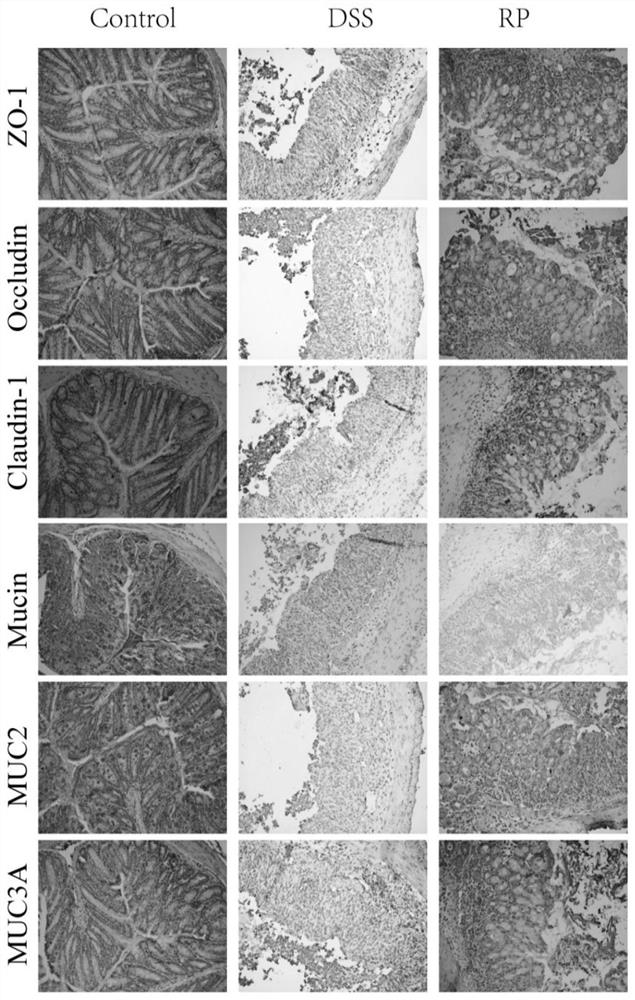
[0055] Example 3 The Repair Effect of PR on Colonic Mucosa Damage
[0056] Immunohistochemical analysis: Paraffin-embedded colon slides (4 μm) were deparaffinized and subjected to antigen retrieval by microwave heating in 10 mM citrate buffer (pH 6.0) for 10 min. After the slides were washed with PBS, they were washed with 3% H 2 o 2 Incubate with goat serum for 10min and 15min at room temperature. The treated slides were added with primary antibodies ZO-1 (1:500, Proteintech, Wuhan, China), occludin (1:500, Abclonal, Wuhan, China), claudin 1 (1:500, Abclonal, Wuhan, China), MUC2 (1:500 , Proteintech, Wuhan, China) and MUC3A (1:500, Boster, Wuhan, China) were incubated overnight at 4°C. Add biotinylated rabbit secondary antibody and incubate for 30 minutes at room temperature. After 30 min incubation with streptavidin-HRP, sections were stained with DAB substrate for 5 min.
[0057] The results of immunochemical staining showed ( figure 2 ), PR treatment maintained prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com