PH sensitive catalyst for catalyzing asymmetric Aldol reaction and preparation method thereof
A catalyst, asymmetric technology, used in catalytic reactions, preparation of organic compounds, catalysts for physical/chemical processes, etc., to achieve high-efficiency catalytic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
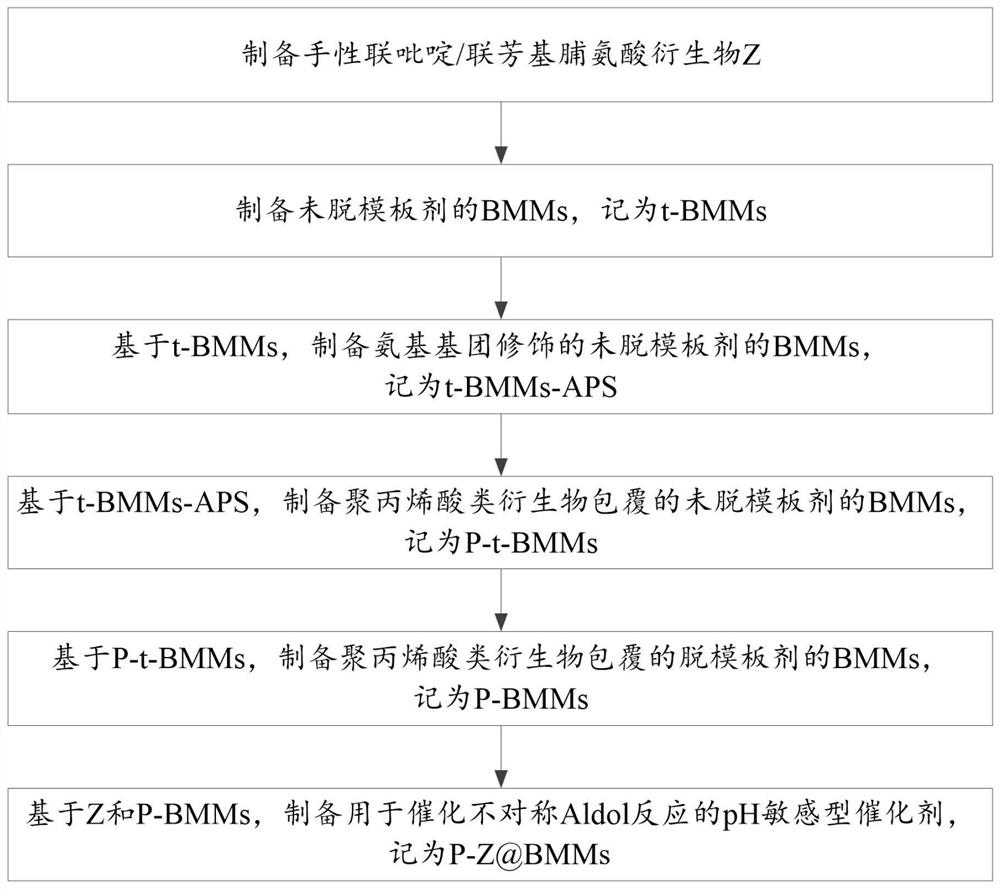
[0061] The invention provides a method for preparing a pH-sensitive catalyst for catalyzing an asymmetric Aldol reaction, comprising:
[0062] Using polyacrylic acid derivatives as the shell and mesoporous silica nanocarriers as the core; the carboxyl groups in the polyacrylic acid derivatives are activated and coupled with the amino groups on the mesoporous silica nanomaterial carrier through amidation Core-shell organic-inorganic nanomaterials were prepared by coating processes such as reaction, and chiral bipyridyl / biarylproline derivatives were loaded on the core-shell organic-inorganic nanomaterials to prepare asymmetric Aldol for catalytic pH-sensitive catalyst for the reaction.
[0063] Such as figure 1 Shown, above-mentioned preparation method specifically comprises:
[0064] Step 1. Preparation of chiral bipyridyl / biarylproline derivative Z; wherein,
[0065] Z was prepared according to the literature (Synlett, 2012, 23, (13): 1990-1994; Chemistry Select, 2020, 5, ...
Embodiment 1
[0087] The invention provides a method for preparing a pH-sensitive catalyst for catalyzing an asymmetric Aldol reaction, comprising:
[0088] Step 1. Prepare Z 1 , the molecular structure is as follows:
[0089]
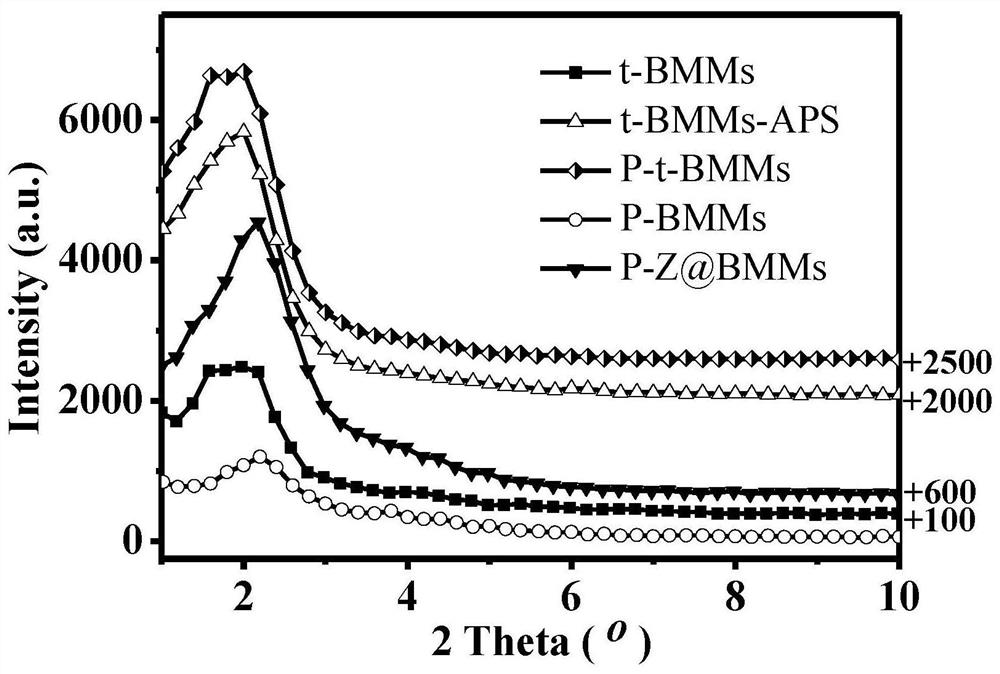
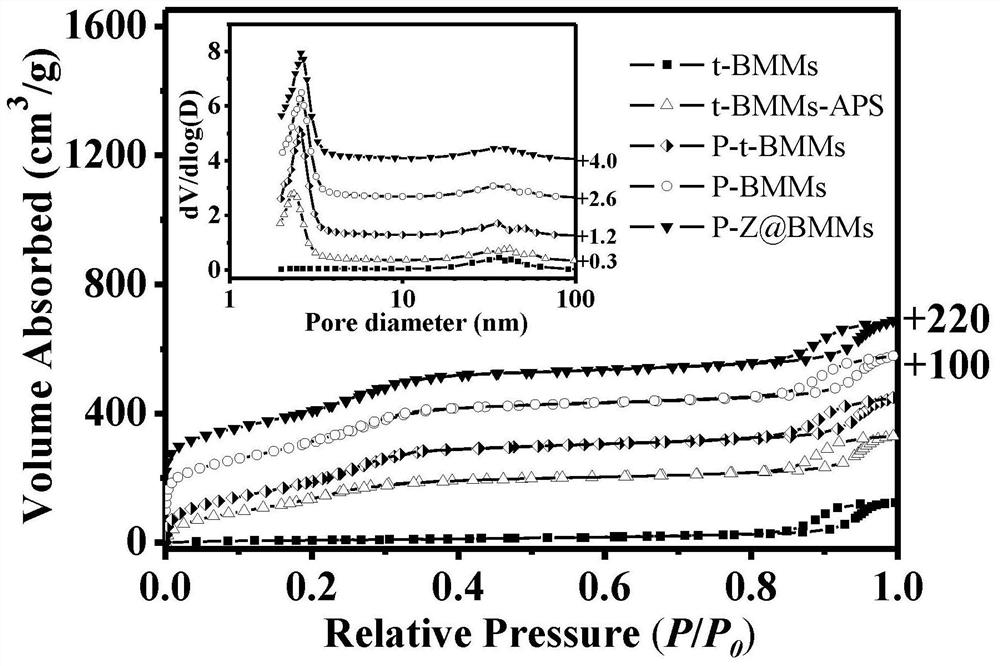
[0090] Step 2. Preparation of BMMs (t-BMMs) without template removal agent CTAB:
[0091] Dissolve 2.62g of CTAB in 105mL of distilled water, stir magnetically until completely dissolved, pipette 8mL of tetraethyl orthosilicate (TEOS) and slowly add to the above solution, then quickly add 3.5mL of 25% ammonia water (NH 3 ·H 2 0), continue to stir until the solution all turns into white gel. The white gel was suction-filtered, washed thoroughly with distilled water, and dried to obtain BMMs without template release, which were designated as t-BMMs.
[0092] Step 3, prepare amino group modified t-BMMs:
[0093] Step 3.1. Weigh 0.5012 g of the t-BMMs sample obtained in step 2 into a round bottom flask, and dry it under vacuum at 150°C for 3 hours;
[0094] Ste...
Embodiment 2
[0113] The invention provides a method for preparing a pH-sensitive catalyst for catalyzing an asymmetric Aldol reaction, comprising:
[0114] Step 1. Prepare Z 2 , the molecular structure is as follows:
[0115]
[0116] Step 2. Preparation of BMMs (t-BMMs) without template removal agent CTAB:
[0117] Dissolve 2.81g of CTAB in 105mL of distilled water, stir magnetically until completely dissolved, pipette 8mL of TEOS and slowly add to the above solution, then quickly add 4.5mL of 25% NH 3 ·H 2 O, continue to stir until the solution turns into a white gel. The white gel was suction-filtered, washed thoroughly with distilled water, and dried to obtain BMMs without template release, which were designated as t-BMMs.
[0118] Step 3, prepare amino group modified t-BMMs:
[0119] Step 3.1. Weigh 0.5012 g of the t-BMMs sample obtained in step 2 into a round bottom flask, and dry it under vacuum at 150°C for 3 hours;
[0120] Step 3.2, disperse the above dried t-BMMs in anh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com