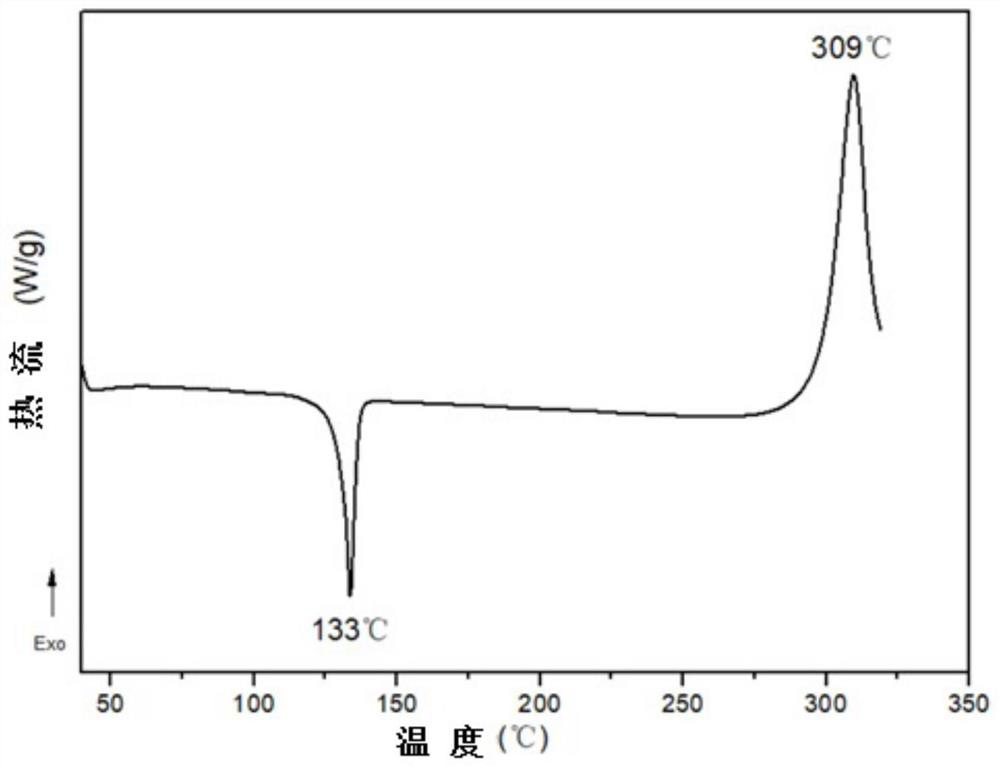
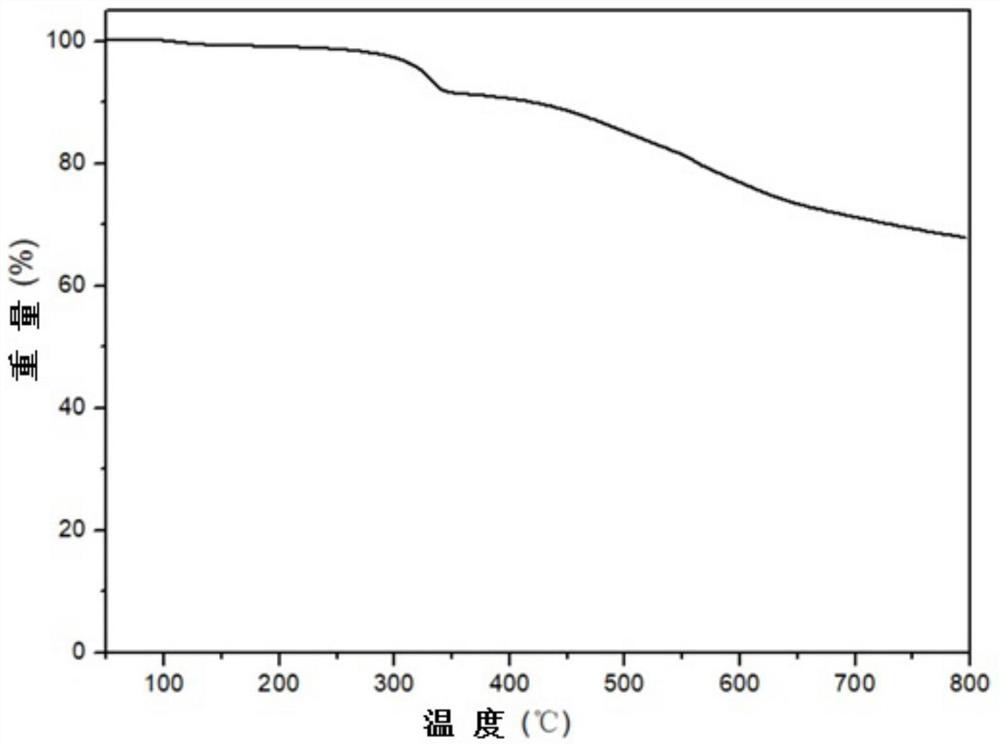
Phthalonitrile monomer containing parylene structure, phthalonitrile resin and preparation method thereof
A technology of phthalonitrile resin and phthalonitrile, applied in the preparation of carboxylic acid nitrile, preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of unsatisfactory dielectric properties of phthalonitrile resin, etc. problems, to achieve the effect of avoiding resin defects, excellent dielectric properties, and high thermal decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
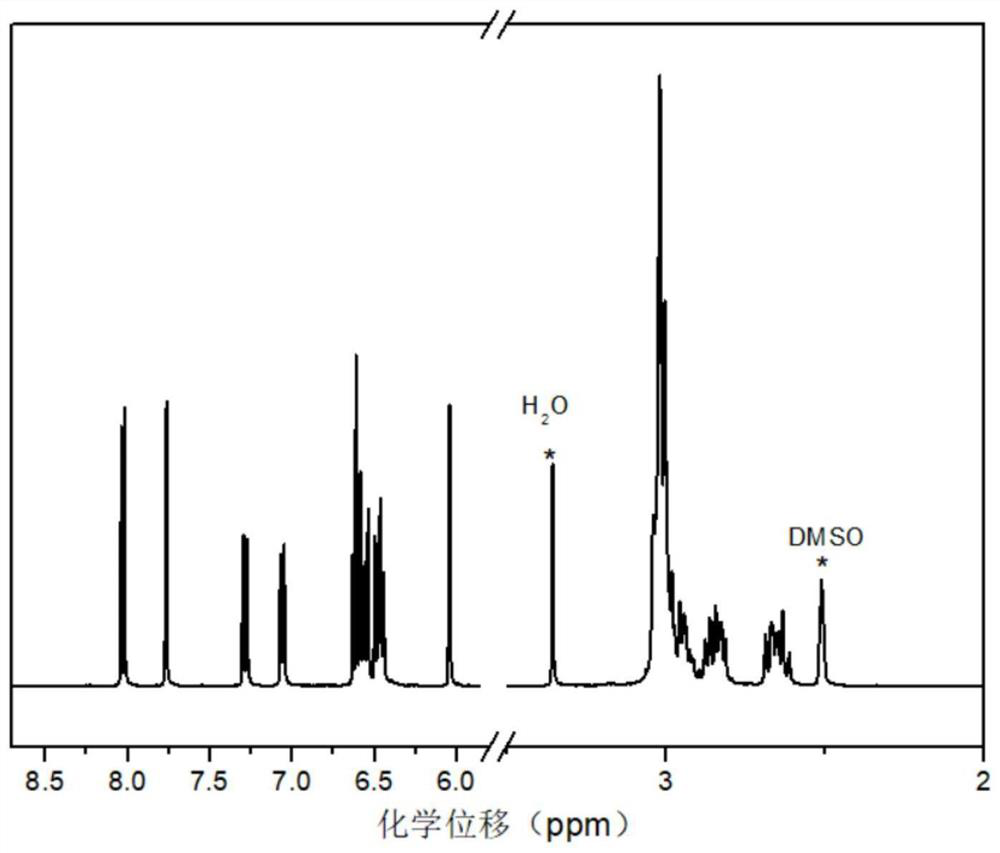
[0062] 1. Preparation of phthalonitrile monomer containing parylene structure
[0063] In this example, phenolic compound 1 containing parylene structure (abbreviated as compound 1: its synthesis process reference: Photochromism of novel chromenes constrained to be part of [2.2] paracyclophane: remarkable'phane'effects on the colored o-quinonoidintermediates ( DOI: 10.1039 / c2nj40575j)) and 4-nitrophthalonitrile as raw materials, using anhydrous potassium phosphate as a catalyst to prepare the phthalonitrile monomer containing parylene structure, the synthetic route is as follows:
[0064]
[0065] The preparation steps are as follows:
[0066] (1) 10g (44.58mmol) of compound 1, 7.72g (44.58mmol) of 4-nitrophthalonitrile and 7.70g (55.73mmol) of anhydrous potassium carbonate were added to the reactor, 84mL of DMSO was added, and the mixture was uniformly obtained The mixture used for the reaction;
[0067] (2) Under nitrogen protection, the mixed solution used for the reac...
Embodiment 2
[0077] 1. Preparation of phthalonitrile monomer containing parylene structure
[0078] In this example, phenolic compound 2 containing parylene structure (referred to as compound 2: its synthesis process reference: Electrophilic Substitution of 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane (DOI : 10.1021 / jo9910536)) and 4-nitrophthalonitrile as raw materials, using anhydrous sodium phosphate as a catalyst to prepare the phthalonitrile monomer containing parylene structure, the synthetic route is as follows:
[0079]
[0080] The preparation steps are as follows:
[0081] (1) Add 16.41g (44.58mmol) of compound 2, 7.72g (44.58mmol) of 4-nitrophthalonitrile and 5.91g (55.73mmol) of anhydrous sodium carbonate to the reactor, then add 84mL of DMAc, mix well obtain a mixed solution for the reaction;
[0082] (2) Under nitrogen protection, the mixed solution used for the reaction was heated to 80°C, and reacted under magnetic stirring conditions for 6 h; then the obtained reac...
Embodiment 3
[0088] 1. Preparation of phthalonitrile monomer containing parylene structure
[0089] In this example, the phenolic compound 3 containing parylene structure (referred to as compound 3, its synthesis process reference: 3Reactions of Nucleophiles with Perfluoro[2.2]paracyclophane (DOI: 10.1021 / jo9014535)) and 4-nitrophthalate Nitrile is a raw material, and anhydrous sodium phosphate is used as a catalyst to prepare the phthalonitrile monomer containing parylene structure, and the synthetic route is as follows:
[0090]
[0091] The preparation steps are as follows:
[0092] (1) 22.02g (44.58mmol) of compound 3, 7.72g (44.58mmol) of 4-nitrophthalonitrile and 5.91g (55.73mmol) of anhydrous sodium carbonate were added to the reactor, then 84mL of DMAc was added, and mixed well obtain a mixed solution for the reaction;
[0093](2) Under the protection of nitrogen, the mixed solution used for the reaction was heated to 80°C and reacted under magnetic stirring conditions for 6 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| dielectric loss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com