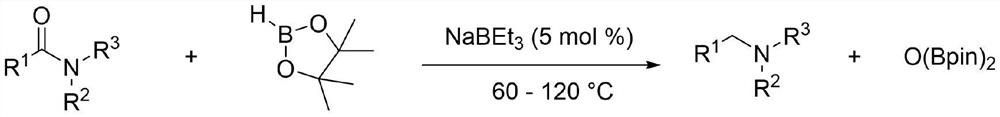
Application method of sodium triethyl borohydride, organic amine compound and preparation method of organic amine compound
A technology of sodium triethylborohydride and sodium ethylborohydride, which is applied in the field of hydroboration reaction, can solve the problems of no industrial production potential, difficulty in preparation, high cost, and high price, and achieve low cost and wide application range , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Sodium triethylborohydride catalyzes the reaction of N,N-dimethylformamide with pinacol borane
[0039] In the glove box, weigh N,N-dimethylformamide (1mmol) and pinacol borane (4.2mmol), take out the flask from the glove box, and add 1mol / L sodium triethylborohydride with a syringe 0.05ml, then heated in an oil bath at 60°C for 12 hours, hydrolyzed the reaction mixture with 1mol / L HCl in ether (10mL), then removed the solvent under reduced pressure, and washed the residue with ethyl acetate (3*5mL) to obtain A pure ammonium salt product, weighing its calculated yield is 96%, and the ammonium salt obtained by separation is dissolved in D 2 O, proceed 1 H NMR and 13 C NMR spectrum test, its result is as follows:
[0040] 1 H NMR (400MHz,D 2 O)δ2.93(s,5H) 13 C NMR (101MHz, D 2 O) δ44.76.
Embodiment 2
[0042] Sodium triethylborohydride catalyzes the reaction of N,N-dimethylacetamide with pinacol borane
[0043] In the glove box, weigh N,N-dimethylacetamide (1mmol) and pinacol borane (4.2mmol), take out the flask from the glove box, and add 1mol / L sodium triethylborohydride with a syringe 0.05ml, then heated in an oil bath at 60°C for 12 hours, hydrolyzed the reaction mixture with 1mol / L HCl in ether (10mL), then removed the solvent under reduced pressure, and washed the residue with ethyl acetate (3*5mL) to obtain A pure ammonium salt product. Weighing calculated yield was 96%, and the ammonium salt obtained by separation was dissolved in D 2 O, proceed 1 H NMR and 13 C NMR spectrum, its result is as follows:
[0044] 1 H NMR (400MHz,D 2 O) δ1.32(t, J=7.3Hz, 3H), 2.87(s, 6H), 3.20(q, J=7.2Hz, 2H).2.93(s, 5H) 13 C NMR (101MHz, D 2 O) δ9.02, 42.01, 53.01.
Embodiment 3
[0046] Sodium triethylborohydride catalyzes the reaction of N-methylacetamide with pinacol borane
[0047]In a glove box, N-methylacetamide (1 mmol), pinacol borane (4.2 mmol) were weighed. Take out the flask from the glove box, add 0.05 ml of 1 mol / L sodium triethylborohydride with a syringe, then heat in an oil bath at 120° C. for 24 hours, and hydrolyze the reaction mixture with 1 mol / L HCl diethyl ether solution (10 mL), Thereafter, the solvent was removed under reduced pressure, and the residue was washed with ethyl acetate (3*5 mL) to obtain a pure ammonium salt product. Weighing calculated yield was 86%, and the ammonium salt obtained by separation was dissolved in D 2 O, carry on 1 H NMR and 13 C NMR spectrum, its result is as follows:
[0048] 1 H NMR (400MHz,D 2 O) δ1.28(t, J=7.5Hz, 3H,), 2.70(s, 3H), 3.09(q, J=7.5Hz, 2H). 13 C NMR (101MHz,D 2 O) δ10.33, 32.12, 44.23.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com