Microcapsule powder stable in gastric acid as well as preparation method and application of microcapsule powder
A technology of microcapsule powder and gastric acid, which is applied in the field of microcapsule powder and its preparation, can solve the problems that NADH or NADPH cannot be decomposed or decomposed, and achieves good stability in an open environment at room temperature, high product embedding rate, and open at room temperature The effect of strong environmental stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
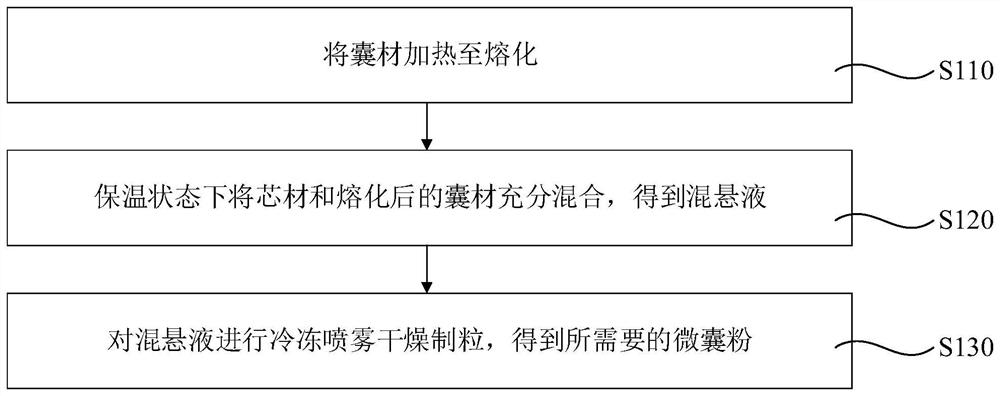
[0054] combine figure 1 , the present invention discloses a method for preparing the above-mentioned microcapsule powder according to one embodiment. The above-mentioned microcapsule powder is prepared by a freeze spray drying method, which includes the following steps:
[0055] S110, heating the capsule material to melt.
[0056] Wherein, the melting point of the capsule material is greater than 42° C., the capsule material will not decompose or dissolve under the action of protease and gastric acid, and the capsule material will decompose under the action of intestinal digestive enzymes.
[0057] S120, fully mixing the core material and the melted capsule material in a heat preservation state to obtain a suspension.
[0058] Preferably, the operation of obtaining the suspension after fully mixing in the heat preservation state is as follows: in the heat preservation state, stirring at a rotation speed of 500 rpm to 1200 rpm for 3 min to 10 min to obtain the suspension.
[...
Embodiment 1
[0095] Preparation of Microcapsule Powder by Freeze Spray Drying
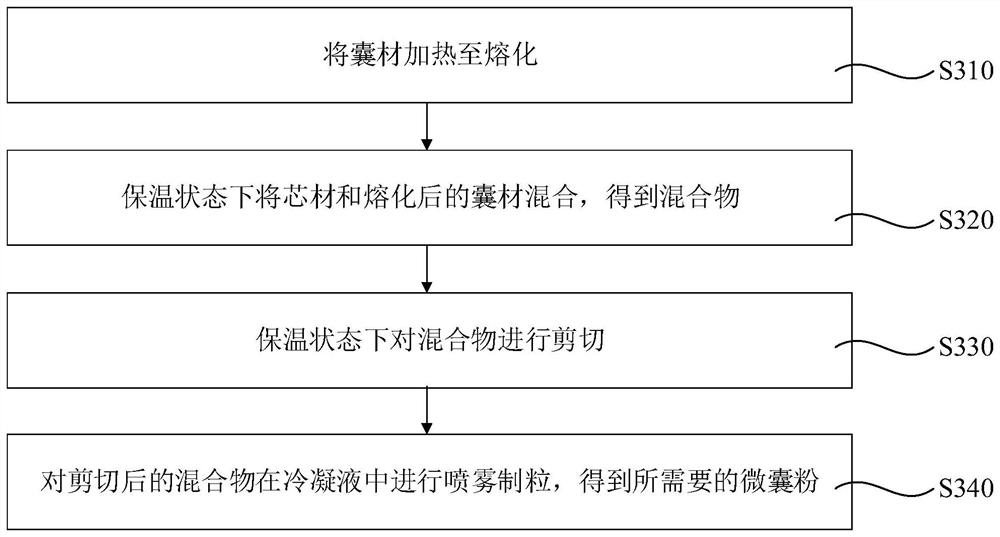
[0096] Accurately weigh 540g of rice bran fatty alkanol in a 1000mL jacketed pot, keep the temperature of the water bath at 80°C until the rice bran fatty alkanol melts, put 60g of NADH into the melted rice bran fatty alkanol while keeping warm, and stir at 500rpm for 10 minutes to fully mix , to obtain a suspension. Use a spray dryer to carry out freeze spray drying and granulation of the suspension, and adjust the working parameters of the spray dryer as follows: inlet air temperature 2°C, outlet air temperature 15°C, cavity middle temperature 5°C, feed rate 1500mL / min, The rice bran fatty alkanol-coated NADH microcapsule powder was obtained, which was designated as sample 1.
Embodiment 2
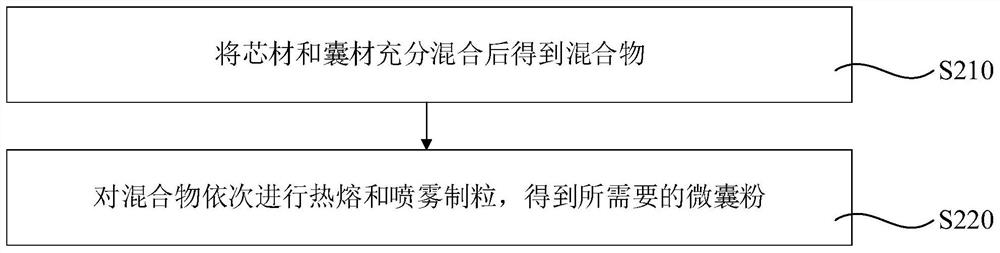
[0098] Preparation of microcapsule powder by hot-melt mixing and spraying method
[0099] Accurately weigh 480g of rice bran fatty alkanol and 120g of NADH and put them into the mixer. Turn on the mixer, the mixing frequency is 25Hz, the mixing time is 60min, and the rice bran fatty alkanol and NADH are fully mixed. After the mixing is completed, put the mixture into the feeding system of the hot melt extruder, heat the mixture at 140°C, and output it to the atomization system for spray embedding granulation. Adjust the rotation speed of the atomizing wheel to 15000rpm, the inlet air temperature to 2°C, the outlet air temperature to 15°C, the temperature in the middle of the cavity to 5°C, and the feed rate to 1500mL / min to obtain rice bran fatty alkanol-coated NADH microcapsule powder, record Make sample 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com