Solid-phase preparation method of pracanatide
A technology for plecanatide and solid-phase preparation, which is applied in the field of solid-phase preparation of plecanatide, can solve the problems of unfavorable industrialized production, complicated operation steps, high waste liquid cost, etc., and achieves easy preparation and purification, and easy avoidance. The effect of reunion and reduction of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
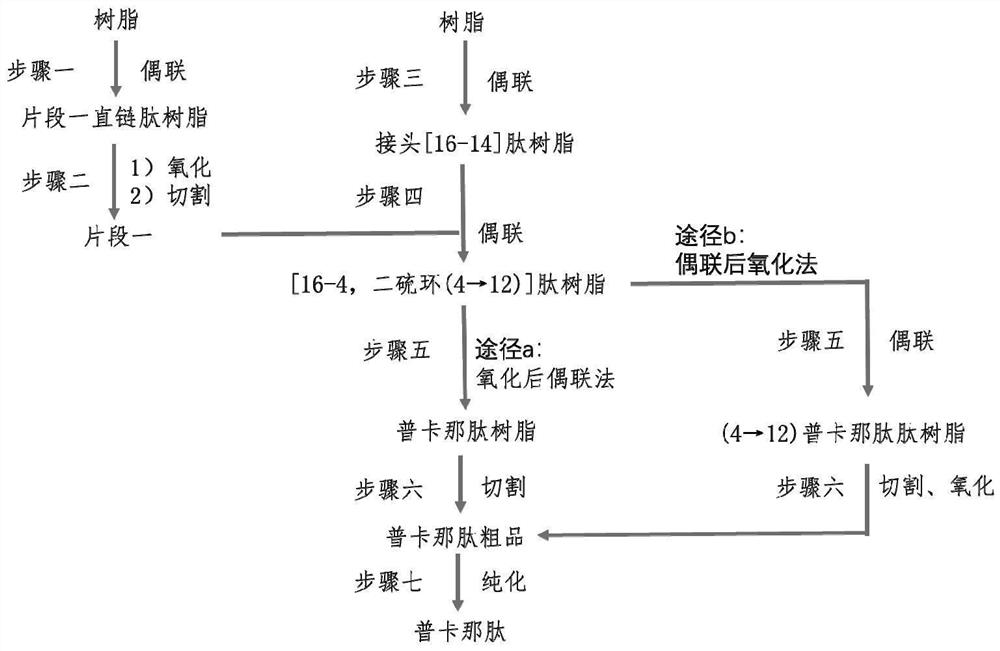
[0163] Example 1: Synthesis of Acm-protected Fragment One (Fragment One [13,4])
[0164] step one:
[0165] Add Wang resin (90.00g, degree of substitution 0.60mmol / g) and 1100mL DCM to the solid-phase reaction bottle successively, and suction filter after swelling to obtain a filter cake. Fmoc-Thr(tBu)-OH (15.51g), DIC (4.92g), HOBt (5.28g), 1100mL DCM were added sequentially at 24±4°C, and the reaction was stirred for 2h under nitrogen protection. Drain, wash 5 times with 300mL*5DCM, and 2 times with 300mL*3DMF. Add 90 mL of acetic anhydride, 90 mL of pyridine, and 900 mL of DMF, and stir for 8 hours under nitrogen protection. After pumping to dryness, 90 mL of acetic anhydride, 90 mL of pyridine, and 900 mL of DMF were added again, and stirred for 8 hours under nitrogen protection. Drained, washed five times with 300mL*5DCM and three times with 350mL*3MeOH. Vacuum drying at room temperature yielded 108.85 g of Fmoc-Thr(tBu)Wang resin. After testing, the degree of substi...
Embodiment 2
[0170] Embodiment 2: the synthesis of the linker peptide resin of Acm protection
[0171] Step three:
[0172] Add Wang resin (30.00g, degree of substitution 0.60mmol / g) and 300mL DCM successively into the solid-phase reaction bottle, and suction filter after swelling to obtain a filter cake. Fmoc-Leu-OH (3.42g), DIC (1.25g), HOBt (1.27g), 180mL DCM were added sequentially at 24±4°C, and the reaction was stirred for 2h under nitrogen protection. Drain, wash 5 times with 100mL*5DCM. Add 30 mL of acetic anhydride, 30 mL of pyridine, and 30 mL of DMF, and stir for 8 hours under nitrogen protection. Drain. Add 30 mL of acetic anhydride, 30 mL of pyridine, and 30 mL of DMF again, and stir for 8 h under nitrogen protection. Drain. 100mL*5DCM was washed five times, and 60mL*3MeOH was washed three times. The filter cake was alternately washed with MeOH and DCM, sucked dry, and dried under vacuum at room temperature to obtain Fmoc-Leu Wang resin. After testing, the degree of sub...
Embodiment 3
[0174] Example 3: Synthesis of [16-4, disulfide bond S-S (4→12)] peptide resin
[0175] Step four:
[0176] Add 40mL of 20% piperidine / DMF solution to the linker peptide resin reaction bottle, mix and stir for 10 minutes, and drain; then add 40mL of 20% piperidine / DMF solution, mix and stir for 10 minutes, and drain. An appropriate amount of DMF was used to wash the filter cake, and the deprotected linker peptide resin to be used was drained. In a clean activation bottle, add fragment one [13-4, disulfide bond S-S (4→12)] (1.00mmol), DIEA (2.1mmol) and 25mL DCM / NMP (1:1, volume ratio), HATU (1.05 mmol) and HOAt (1.05 mmol) were sequentially added at 5±4° C., and activated under nitrogen for 2 minutes. Under nitrogen protection, transfer the activation solution to the deprotected linker peptide resin reaction vial. React for 2 hours at 24±4°C under nitrogen, and drain; add Fragment 1 [13-4, disulfide bond S-S(4→12)] (1.00mmol), DIEA (2.1mmol) and 25mL DCM to a clean activati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com