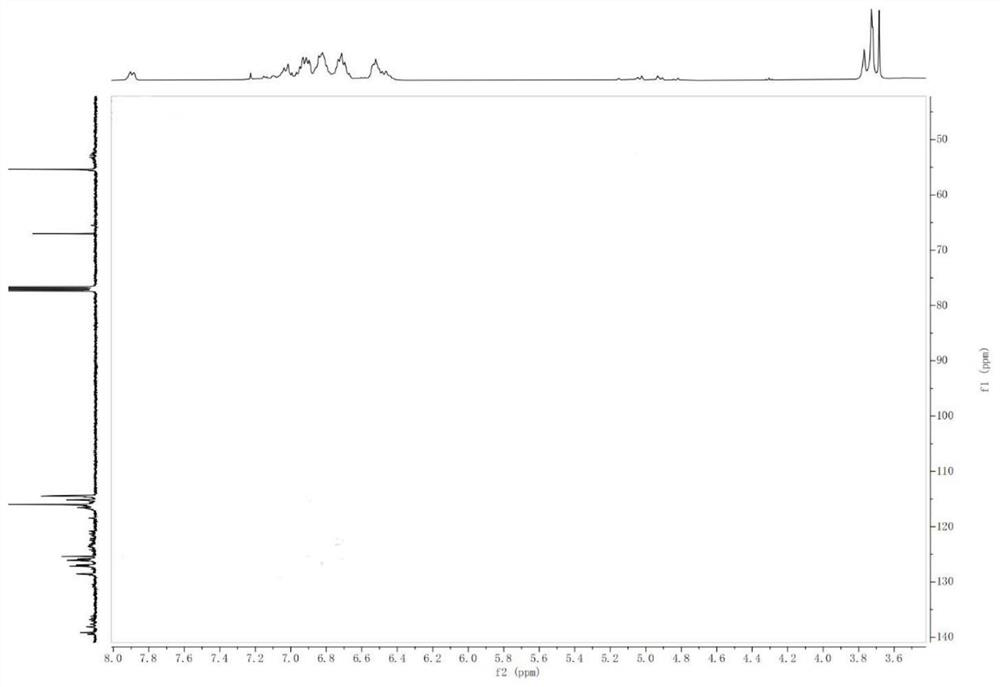
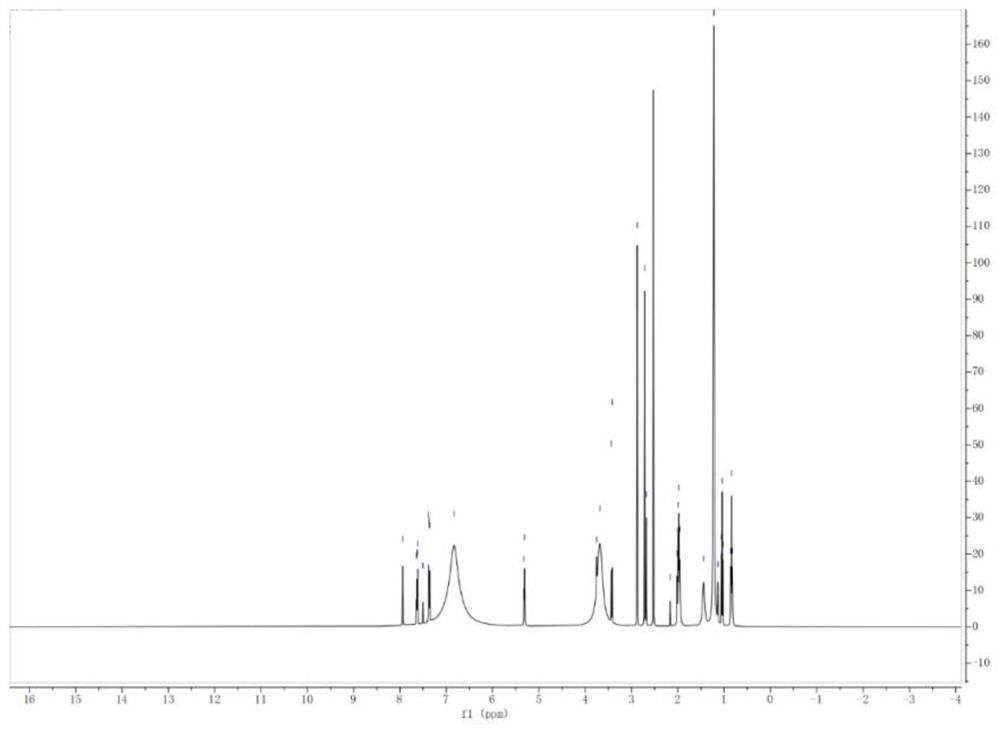
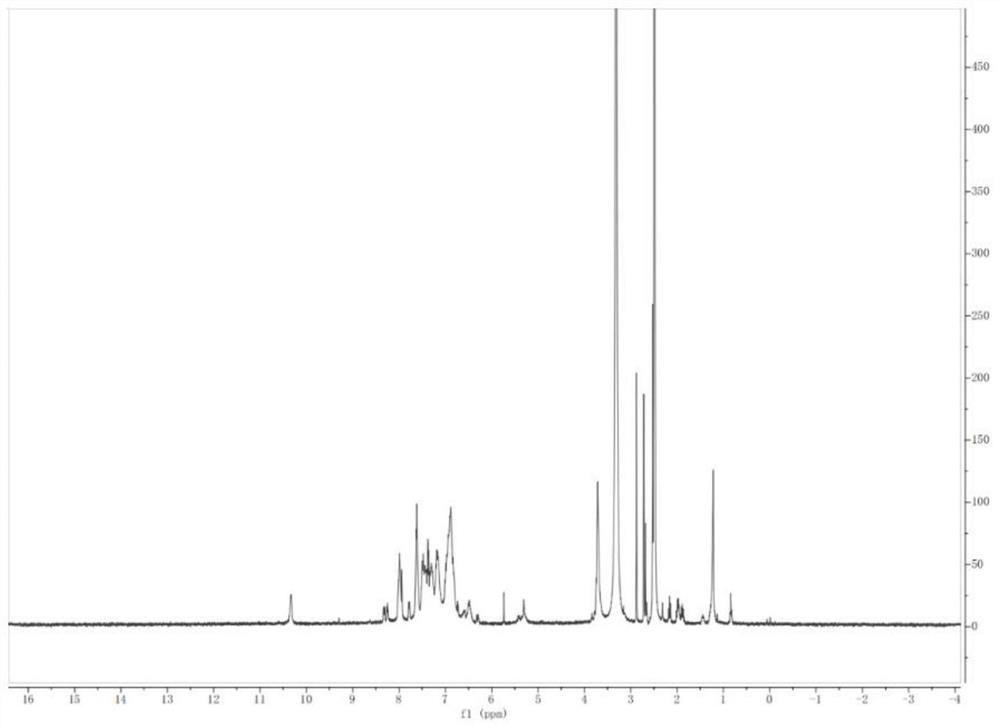
Triptycene-centered methoxytriphenylamine-containing polyamide as well as a preparation method and application thereof
A technology of methoxytriphenylamine and triptycene, applied in photovoltaic power generation, instruments, optics, etc., can solve the problems of triptycene's narrow application range, low solubility, and no electrochromic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0079] Specific Embodiment 1: In this embodiment, triptycene-centered methoxytriphenylamine-containing polyamides are triptycene-centered methoxy-containing triphenylamine polyamides P1, triptycene-centered methoxytriphenylamine-containing polyamides Triphenylamine-based polyamide P2 or triptycene-based polyamide P3 containing methoxytriphenylamine;
[0080] Among them, the structural formula of polyamide P1 containing triptycene as the center and containing methoxytriphenylamine is as follows:
[0081] ,
[0082] In the formula, n is an integer of 12 to 16;
[0083] Among them, the structural formula of triptycene-containing polyamide P2 containing methoxytriphenylamine is as follows:
[0084] ,
[0085] In the formula, n is an integer of 12 to 16;
[0086] Among them, the structural formula of polyamide P3 containing triptycene as the center and containing methoxytriphenylamine is as follows:
[0087] ,
[0088] In the formula, n is an integer of 12-16.
[0089]...
specific Embodiment approach 2
[0094] Specific implementation mode 2: In this implementation mode, the preparation method of triptycene-based polyamide containing methoxy triphenylamine is as follows:
[0095] 1. Synthesis of 2,6,14-triiodotriptycene monomer:
[0096] ①. Add concentrated nitric acid to triptycene, heat to 80°C for reaction, after the reaction, cool to room temperature to obtain mixture A, then pour mixture A into ice water to stir and precipitate, after the precipitation is complete, suction filter, wash with water, vacuum Drying, followed by separation and purification to obtain compound M1;
[0097] ② Put compound M1 and Reney-Ni into anhydrous tetrahydrofuran, then add hydrazine hydrate under nitrogen atmosphere, heat to 65°C for reaction, cool to room temperature after the reaction, filter, and rotate the filtrate to obtain compound M2;
[0098]③. Add compound M2 to hydrochloric acid solution, and then add NaNO dropwise at 0-5°C 2 aqueous solution, stirred until clear, then added KI a...
specific Embodiment approach 3
[0141] Embodiment 3: The difference between this embodiment and Embodiment 2 is that in both steps 1 and 3, TLC analysis is used to monitor the reaction process. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com