Double-loading type PEGylated lipidosome and application thereof
A liposome and plastid technology, applied in the field of new pharmaceutical formulations, can solve problems such as clinical application limitations, and achieve the effects of uniform particle size, high encapsulation efficiency and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
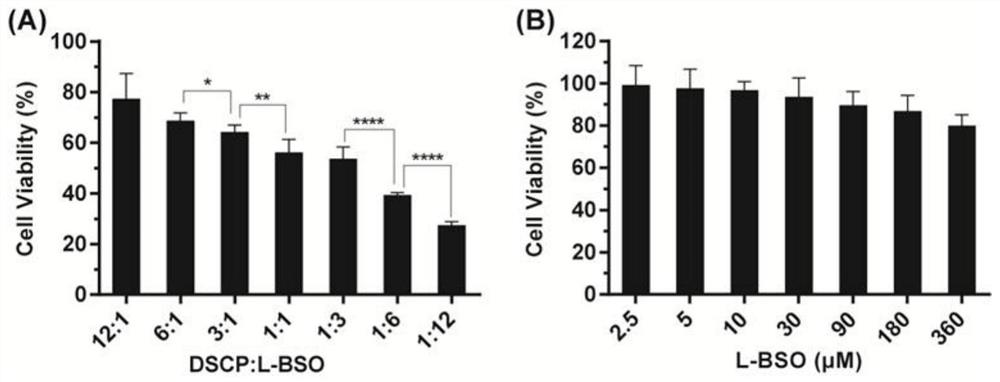
[0027] Screening of DSCP and L-BSO feed ratio:
[0028] Prepare mixed solutions of DSCP and L-BSO in different ratios: Weigh an appropriate amount of DSCP and L-BSO, dissolve them in deionized water, and dissolve them in different molar ratios (12:1, 6:1, 3:1, 1:1, 1:1) 3. 1:6, 1:12) Prepare a set of mixed solutions of DSCP and L-BSO.
[0029] The CCK-8 experiment was used to investigate the effect of the mixed solution of different proportions of DSCP and L-BSO on the survival rate of 4T1 / DDP cells: the mixed solution of DSCP and L-BSO was filtered and sterilized through a 0.22 μm sterile filter membrane in a sterile operating table , diluted with RPMI-1640 medium containing 10% fetal bovine serum. Take 4T1 / DDP cells in the logarithmic growth phase and dilute them to 3×10 with RPMI-1640 medium containing 10% fetal bovine serum 4 Cells / cells / mL were inoculated in a 96-well plate, 100 μL per well, placed at 37°C, 5% CO 2 Cultivate in the incubator for 12h. The medium was di...
Embodiment 2
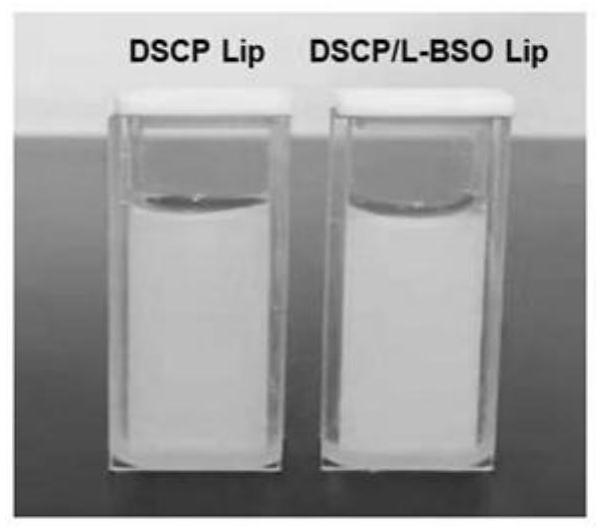
[0033] Preparation and appearance comparison of DSCP / L-BSO Lip and control group DSCP Lip:
[0034] According to the results of Example 1, 4 mg of DSCP and 20 mg of L-BSO were weighed and dissolved in 4 mL of deionized water to form a mixed solution of DSCP and L-BSO.
[0035] Liposomes of target size (for example, less than 200 nm) are prepared by film hydration-extrusion method, and free drug is removed by ultrafiltration centrifugation. Film hydration-extrusion method is as follows: Weigh egg yolk lecithin 127.77mg, cholesterol 42.53mg and DSPE-mPEG 2K 42.51 mg was placed in a 250mL eggplant-shaped bottle, and 20mLCH was added 2 Cl 2 Form a liposome solution, remove CH by rotary evaporation 2 Cl 2, to make a uniform liposome film, dry it in vacuum at 25°C, add a mixed solution of DSCP and L-BSO (deionized aqueous solution containing 4mgDSCP and 20mg L-BSO), stir magnetically for 5min, then add 4mL deionized water, stir magnetically After 2.5h, crude DSCP / L-BSO Lip was ...
Embodiment 3
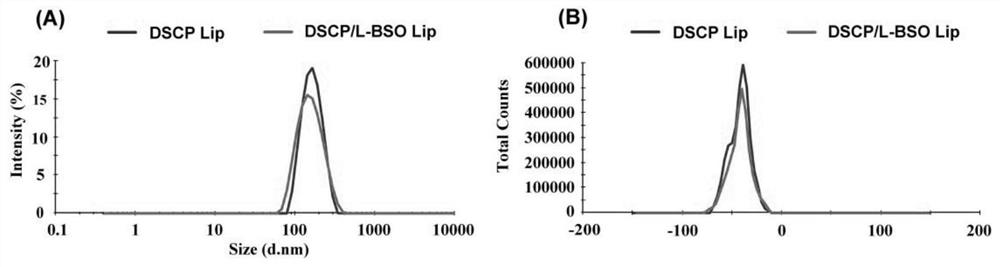
[0039] The particle size and zeta potential of the DSCP / L-BSO Lip and DSCP Lip prepared in Example 2 were measured by a particle size analyzer. The DSCP Lip and DSCP / L-BSO Lip prepared in Example 2 are diluted 10 times with deionized water, and the MalvernZetasizer particle size analyzer is used to measure particle size distribution and Zeta potential. The results are as follows: image 3 As shown in Table 1, Table 1 is the specific numerical values of particle size distribution and Zeta potential.
[0040] image 3 And the results in Table 1 show that the average particle size of DSCP / L-BSO Lip and DSCP Lip are 143.66±1.25nm and 154.88±2.67nm respectively, Zeta potential is respectively-42.10±0.12mV and-42.84±0.07mV, PDI is respectively 0.13±0.01 and 0.12±0.01.
[0041] Table 1
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com