Chloasma-fading composition and preparation method thereof
A composition and a technology for chloasma, applied in the field of medicine, can solve problems such as unsatisfactory treatment effect, and achieve the effect of convenient administration and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
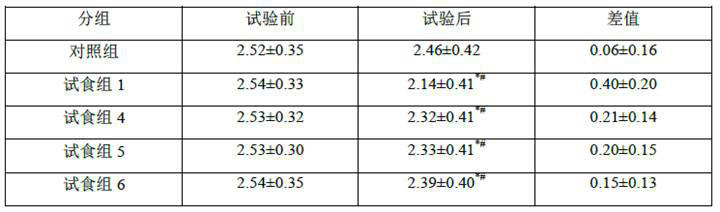
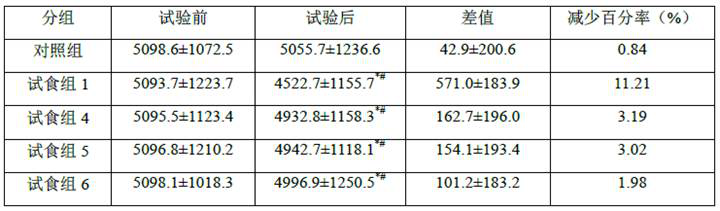
Image
Examples
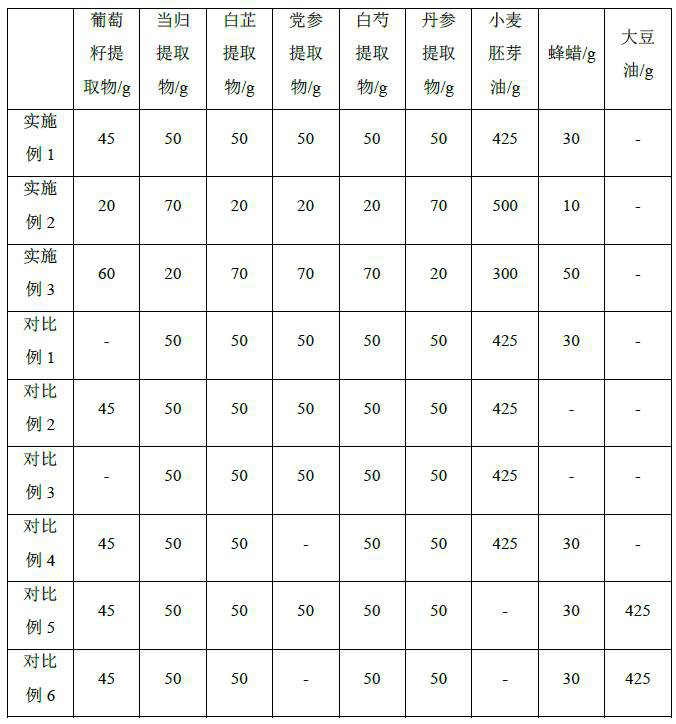
preparation example 1
[0034] Preparation Example 1 Preparation of Radix Angelicae Radix Extract
[0035] The preparation method of Angelica dahurica extract is as follows:
[0036] Pulverize the medicinal material of Angelica dahurica, pass through a 10-mesh sieve, add 8 times the amount of 30% ethanol by volume, soak at room temperature for 1 hour, and extract dynamically for 6 hours in countercurrent to obtain the extract;
[0037] After the extract was filtered, it was centrifuged to obtain the supernatant;
[0038] The supernatant was concentrated under reduced pressure to a solid content of 1.35 g / ml to obtain a concentrate;
[0039] The concentrate is belt-dried and the temperature is controlled at 105-110° C. to obtain a dry product, which is pulverized to obtain the Angelica dahurica extract.
Embodiment 1
[0041] A preparation method of a composition for lightening chloasma, comprising the steps of:
[0042] A. Weigh grape seed extract, Angelica sinensis extract, Angelica dahurica extract, Codonopsis pilosula extract, Radix Paeoniae Alba extract, Salvia miltiorrhiza extract, beeswax, pulverize, sieve, then add wheat germ oil, mix, and make capsule core;
[0043] B. Add the capsule core to the capsule material, and press it into soft capsules of 0.5 g each; the preparation method of the capsule material includes: mixing and dissolving the auxiliary materials gelatin, glycerin, and water in a volume ratio of 1:0.6:1.
[0044] C. The soft capsules are washed, dried, screened, inner-packed and outer-packed in turn to make a finished product.
Embodiment 2
[0046]A preparation method of a composition for lightening chloasma, comprising the steps of:
[0047] A. Weigh grape seed extract, Angelica sinensis extract, Angelica dahurica extract, Codonopsis pilosula extract, Radix Paeoniae Alba extract, Salvia miltiorrhiza extract, beeswax, pulverize, sieve, then add wheat germ oil, mix, and make capsule core;
[0048] B. Add the capsule core to the capsule material, and press it into soft capsules of 0.4 g each; the preparation method of the capsule material includes: mixing and dissolving the auxiliary materials gelatin, glycerin, and water in a volume ratio of 1:0.4:1.
[0049] C. The soft capsules are washed, dried, screened, inner-packed and outer-packed in turn to make a finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com