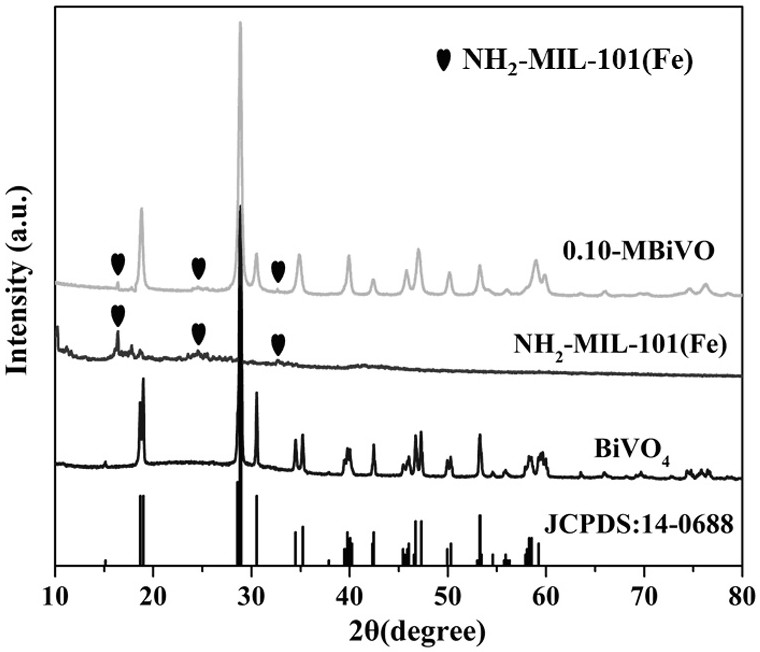
Preparation method and application of photocatalyst for selectively reducing nitrate into N2
A technology of photocatalyst and nitrate, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc. Catalytic activity, effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The invention provides a selective reduction of nitrate to N 2 The photocatalyst preparation method comprises the steps:
[0036] Step (1): preparation of heterojunction photocatalyst;
[0037] NH 2 -MIL-101(Fe) preparation: 6 mmol FeCl 3 ·6H 2 O was dissolved in 72 mL of N,N-dimethylformamide solution, and then 3 mmol of 2-aminoterephthalic acid was added, and ultrasonicated for 20 min until the 2-aminoterephthalic acid was completely dissolved. The solution was transferred to a 120 mL polytetrafluoroethylene reactor and heated at 110 °C for 20 h. After the reaction, the reaction kettle was naturally cooled to room temperature, and the crude product was separated by pressure release and filtration. The crude product was washed and centrifuged several times with N, N-dimethylformamide solution and absolute ethanol solution to obtain the final product. The final product was purified in a blast drying oven at 150 °C for 8 h. The obtained dark brown powder is NH 2 ...
Embodiment 2
[0050] The difference between this embodiment and embodiment 1 is: the photocatalyst prepared in the step (1) is NH 2 -MIL-101(Fe) added with 5%, 10% and 15% NH 2 -MIL-101(Fe) / BiVO 4 heterojunction photocatalysts.
[0051] Step (1): NH 2 -MIL-101(Fe) / BiVO 4 Preparation of heterojunction photocatalysts;
[0052] NH 2 -MIL-101(Fe) preparation: 6 mmol FeCl 3 ·6H 2 O was dissolved in 72 mL of N,N-dimethylformamide solution, and then 3 mmol of 2-aminoterephthalic acid was added, and ultrasonicated for 20 min until the 2-aminoterephthalic acid was completely dissolved. The solution was transferred to a 120 mL polytetrafluoroethylene reactor and heated at 110 °C for 20 h. After the reaction, the reaction kettle was naturally cooled to room temperature, and the crude product was separated by pressure release and filtration. The crude product was washed and centrifuged several times with N, N-dimethylformamide solution and absolute ethanol solution to obtain the final product....
Embodiment 3
[0060] The difference between this example and Example 1 is that the hole trapping agents used in step (2) are 80 mmol / L formic acid, methanol, and oxalic acid, respectively.
[0061] Step (1): preparation of heterojunction photocatalyst;
[0062] NH 2 -MIL-101(Fe) preparation: 6 mmol FeCl 3 ·6H 2 O was dissolved in 72mL of N,N-dimethylformamide solution, and then 3 mmol of 2-aminoterephthalic acid was added, and ultrasonicated for 20 min until the 2-aminoterephthalic acid was completely dissolved. The solution was transferred to a 120 mL polytetrafluoroethylene reactor and heated at 110 °C for 20 h. After the reaction, the reaction kettle was naturally cooled to room temperature, and the crude product was separated by pressure release and filtration. The crude product was washed and centrifuged several times with N, N-dimethylformamide solution and absolute ethanol solution to obtain the final product. The final product was purified in a blast drying oven at 150 °C for 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com