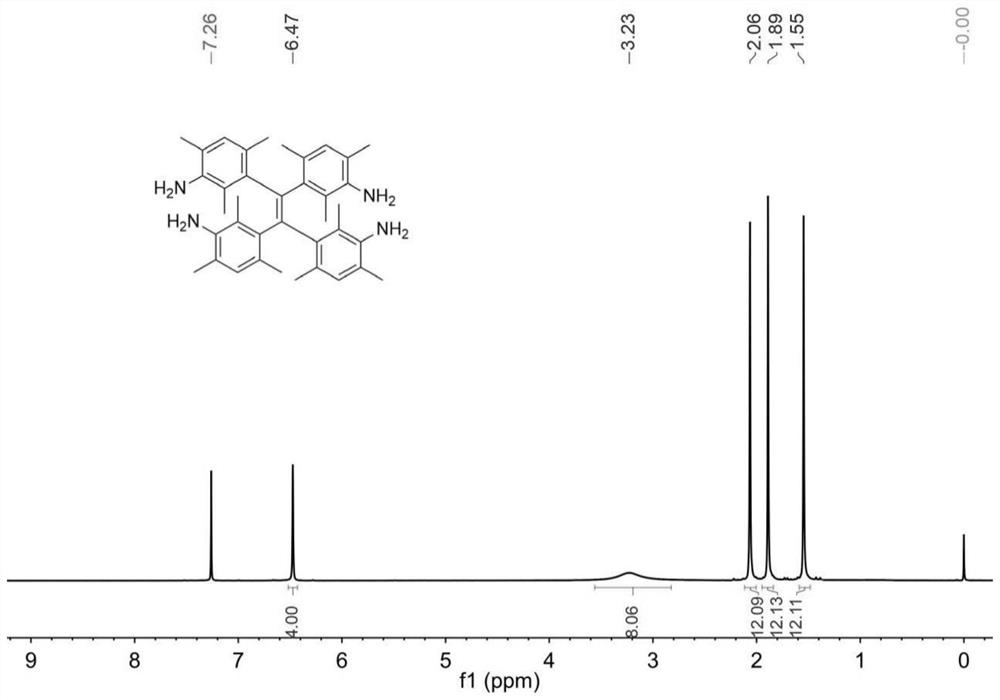
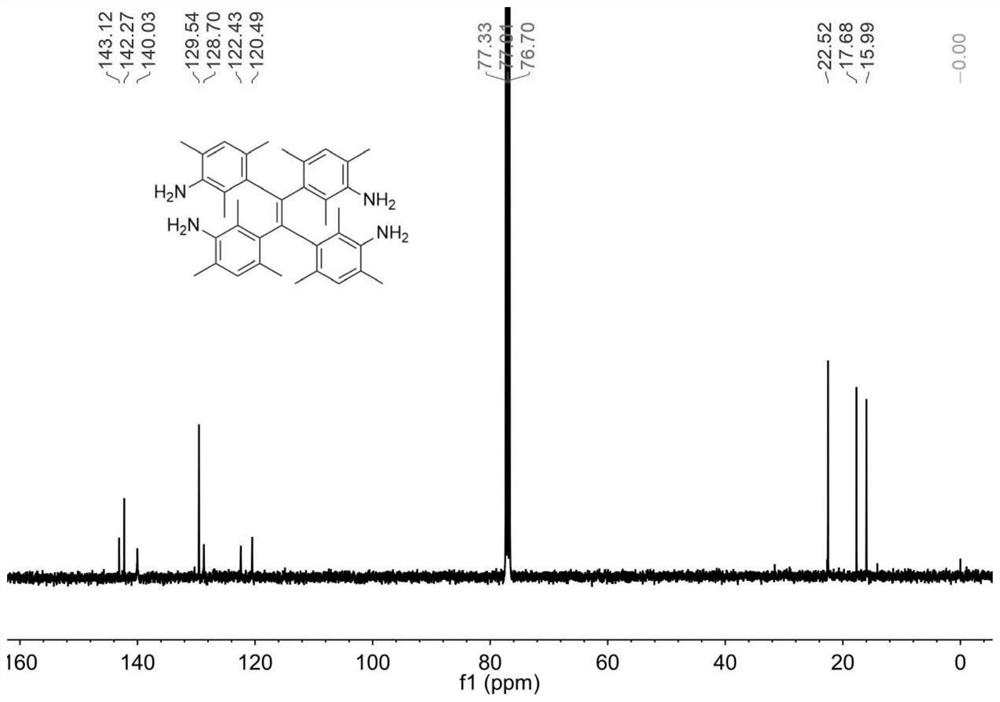
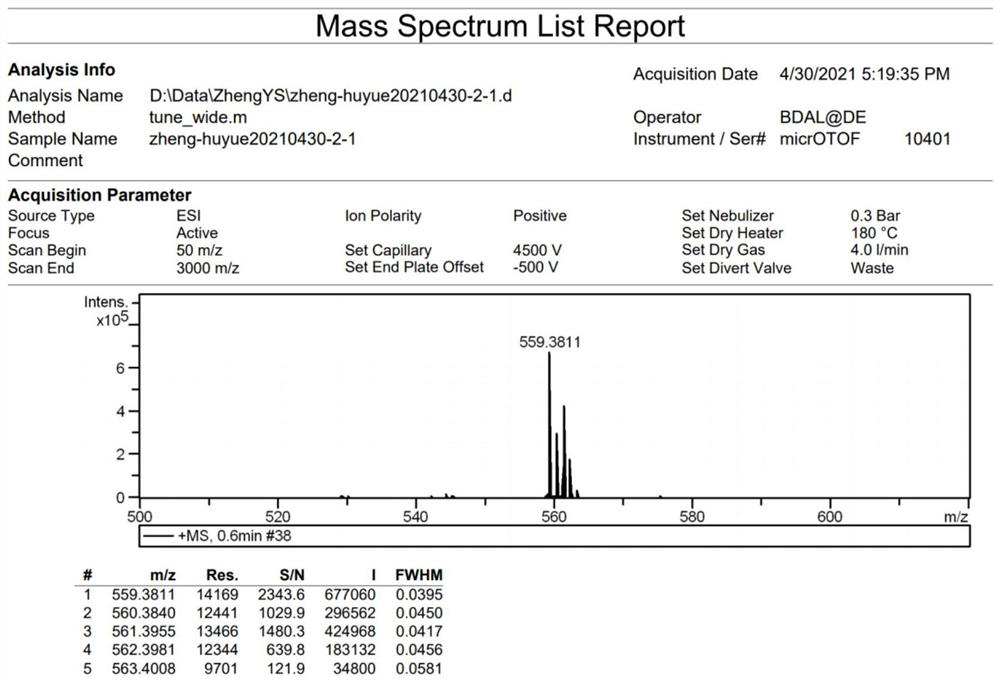
A sterically hindered tetraphenylethylene spirochete emitting dark blue fluorescence and its synthesis method
A technology of left helix and acetic acid, applied in the field of chemistry, can solve the problems of not easy chemical modification, and the inability of tetraphenyl spirochetes to emit dark blue fluorescence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] AIE propeller type molecule I is synthesized by the following route:
[0046]
[0047] i)98%H 2 SO 4 / AcOH / 60°C.
[0048] ii) SOCl 2 / pyridine / benzene / reflux.
[0049] iii) 245nm light / cyclohexane / N 2 / 25°C.
[0050] iv) Fuming HNO 3 / CH 2 Cl 2 / 0°C.
[0051] v) NH 2 NH 2 ·H 2 O / Pd-C / EtOH / reflux.
[0052] Add the compound of formula II (50-200g), glyoxylic acid (10-100g) and acetic acid (100-500mL) into a round bottom flask in sequence, and heat the reaction mixture to reflux for reaction, then add concentrated sulfuric acid 100-500mL under ice-bath cooling , heated and stirred for 10-50 hours, filtered, and the filtrate was extracted with dichloromethane. After the extract was washed with water and dried over anhydrous sodium sulfate, it was evaporated to dryness, and the obtained solid was recrystallized from petroleum ether to obtain a white compound of formula III with a yield of 80%.
[0053] Add the compound of formula III (10-30g), anhydrous ben...
Embodiment 2
[0061] Prepare a high-pressure liquid chromatography column with chirality, and the mobile phase is an ethanol-dichloromethane mixed solvent with a volume ratio of 80:20, and the compound of formula I (R 1 = H, R 3 =NH 2 , R 2 =CH 3 ) was split into two peaks of left helicoid and right helicoid, the retention time of peak 1 was 6.784min, and the retention time of peak 2 was 9.565min. The percent enantiomeric excess of the two single helices of peak 1 and peak 2 is greater than 99%. Measured by single crystal x-ray diffraction, the compound of formula I contains an equal amount of left-helical body and right-helical body, which is a racemate; the corresponding molecular structure of peak 1 is the left-helical body in the compound of formula I, which is called M-I; peak 2 is a right-helical body, called P-I. For the single crystal structure of the compound of formula I and M-I, see Figure 4 and Figure 5 .
Embodiment 3
[0063] In tetrahydrofuran at a concentration of 4 mg / mL, M-I(R 1 = H, R 3 =NH 2 , R 2 =CH 3 )Specific rotation[α] 20 D =+1173°; P-I(R 1 = H, R 3 =NH 2 , R 2 =CH 3 )Specific rotation[α] 20 D =-1203°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com