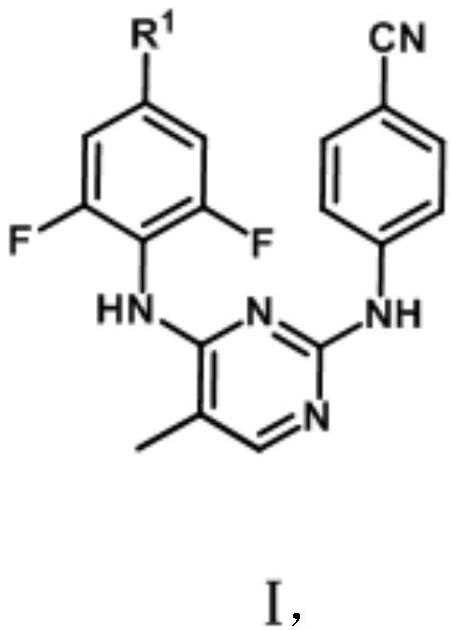
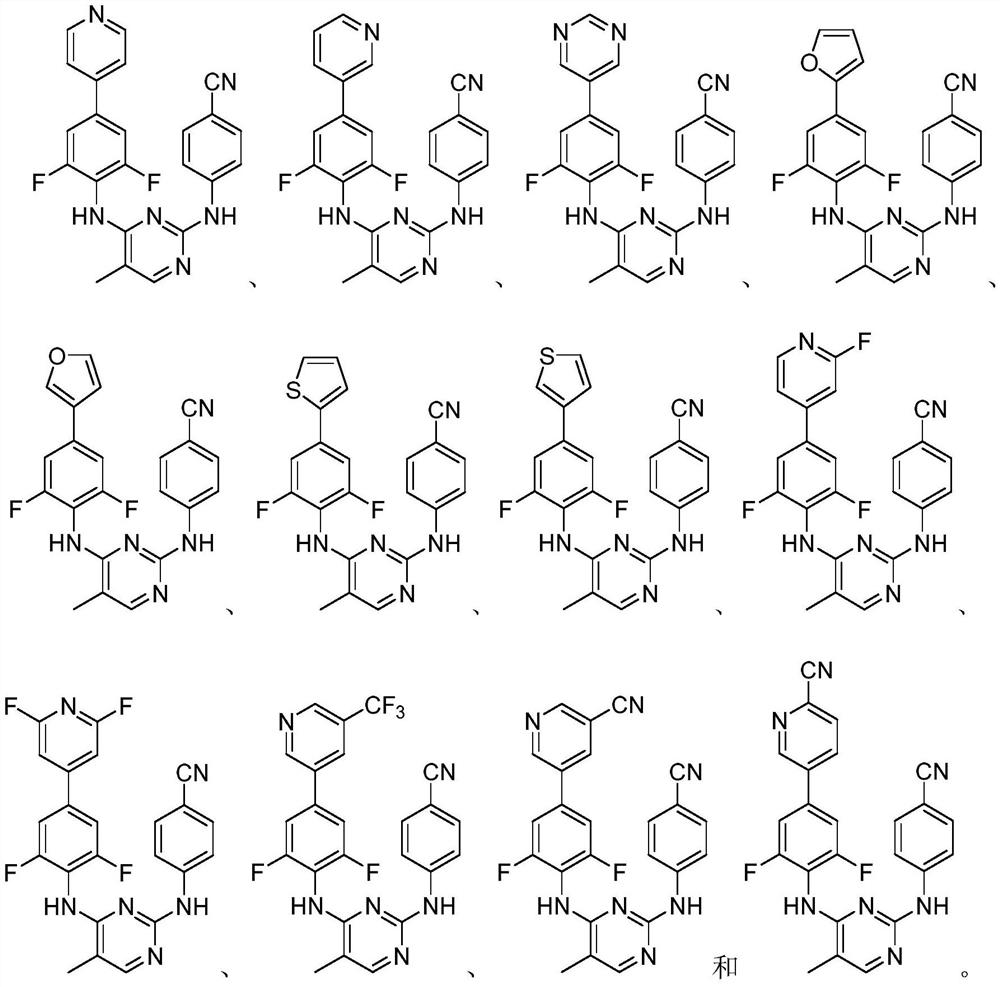
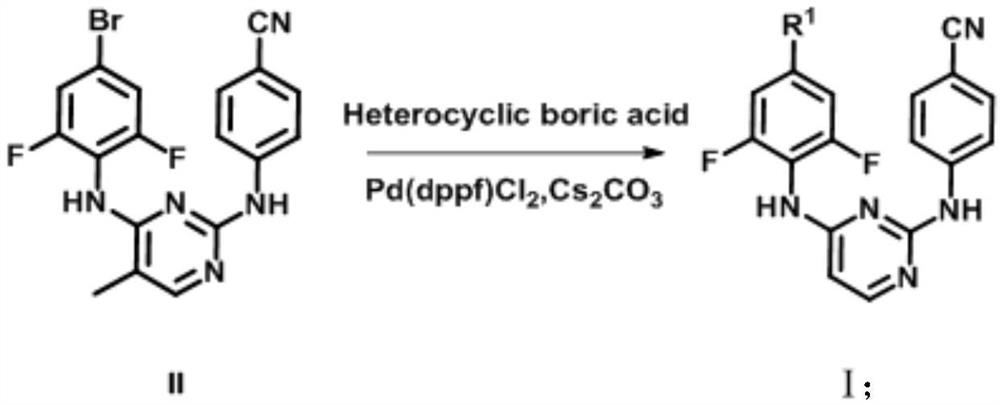
Biphenyl diaryl methyl pyrimidine derivative containing aromatic heterocyclic structure, and preparation method thereof
A technology of biphenyl diarylmethylpyrimidine and aromatic heterocycle, which is applied in the field of biphenyldiarylmethylpyrimidine derivatives and their preparation, and can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of target product (Ia)
[0035]
[0036] At room temperature, 4-((4-((4-bromo-2,6-difluorophenyl)amino)5-methylpyrimidine-2-)amino)benzonitrile (1.0mmol), cesium carbonate ( 1.0mmol), Pd(dppf)Cl 2 (0.01mmol) and 4-pyridineboronic acid (1.2mmol) were added into 1,4-dioxane (6mL), and the 2 Replaced three times, adjusted the reaction temperature to 110°C, and stirred for 4h. As detected by TLC (PE / EA=1 / 1), the starting material disappeared and the reaction was complete. Adjust the reaction temperature to room temperature, wash with saturated sodium sulfite solution (20mL×2), saturated sodium carbonate solution (20mL×2), water (20mL×2), saturated brine (20mL×2) successively, and wash the organic phase with anhydrous sulfuric acid Sodium dry overnight. Filtration, concentration, and methanol recrystallization gave a solid—the target compound (Ia).
[0037] Characterization results of the target product (Ia): white powdery solid; yield 7...
Embodiment 2
[0038] Embodiment 2: the preparation of target product (Ib)
[0039]
[0040] At room temperature, 4-((4-((4-bromo-2,6-difluorophenyl)amino)5-methylpyrimidine-2-)amino)benzonitrile (1.0mmol), cesium carbonate ( 2.0mmol), Pd(dppf)Cl 2 (0.01mmol) and 3-pyridineboronic acid (1.2mmol) were added into 1,4-dioxane (5mL), and the 2 Replaced three times, adjusted the reaction temperature to 150°C, and stirred for 4h. As detected by TLC (PE / EA=1 / 1), the starting material disappeared and the reaction was complete. Adjust the reaction temperature to room temperature, wash with saturated sodium sulfite solution (20mL×2), saturated sodium carbonate solution (20mL×2), water (20mL×2), saturated brine (20mL×2) successively, and wash the organic phase with anhydrous sulfuric acid Sodium dry overnight. Filtration, concentration, and methanol recrystallization gave a solid—the target compound (Ib).
[0041] Characterization results of the target product (Ir): white powdery solid; yield 8...
Embodiment 3
[0042] Embodiment 3: the preparation of target product (Ic)
[0043]
[0044] At room temperature, 4-((4-((4-bromo-2,6-difluorophenyl)amino)5-methylpyrimidine-2-)amino)benzonitrile (1.0mmol), cesium carbonate ( 2.0mmol), Pd(dppf)Cl 2 (0.01mmol) and 5-pyrimidineboronic acid (1.0mmol) were added to 1,4-dioxane (6mL), and the 2 Replaced three times, adjusted the reaction temperature to 80°C, and stirred for 4h. As detected by TLC (PE / EA=1 / 1), the starting material disappeared and the reaction was complete. Adjust the reaction temperature to room temperature, wash with saturated sodium sulfite solution (20mL×2), saturated sodium carbonate solution (20mL×2), water (20mL×2), saturated brine (20mL×2) successively, and wash the organic phase with anhydrous sulfuric acid Sodium dry overnight. Filtration, concentration, and methanol recrystallization gave a solid—the target compound (Ic).
[0045] Characterization results of the target product (Ic): white powdery solid; yield 93...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com