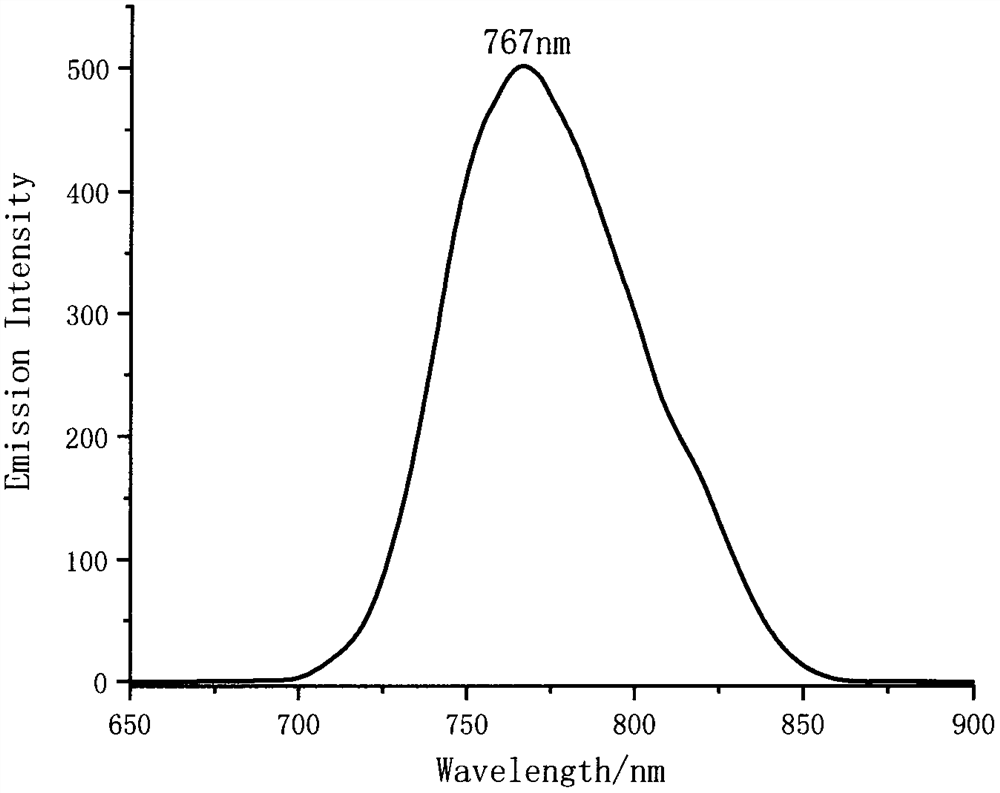
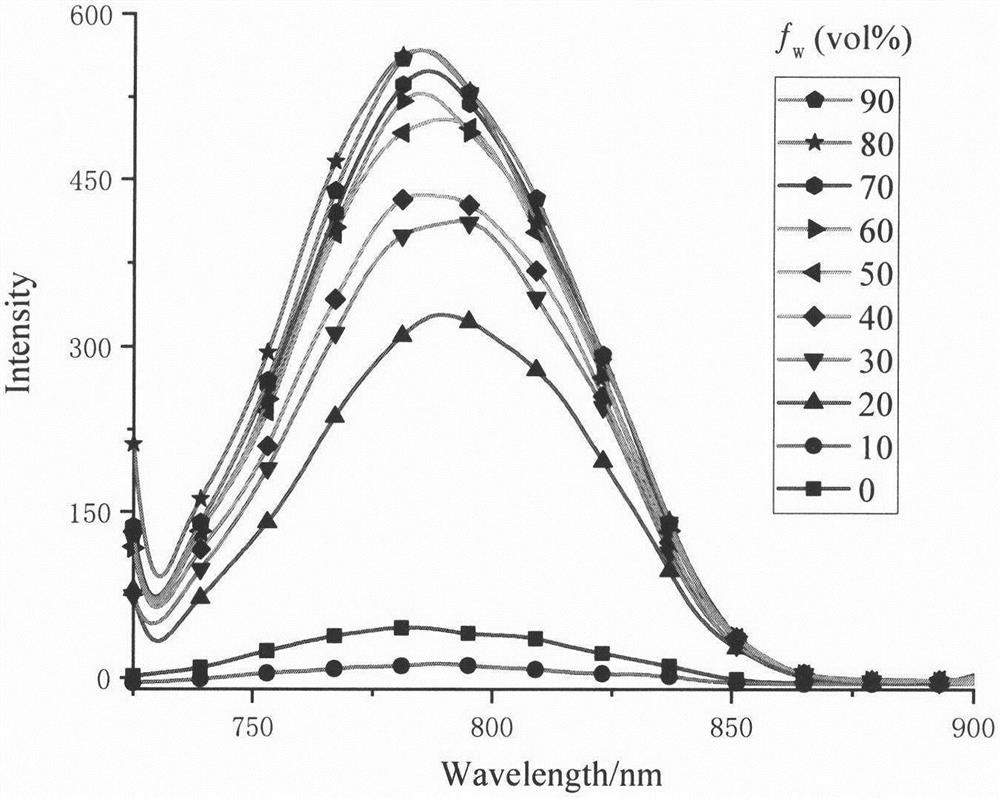
Double-BODIPY near-infrared fluorescent dye with AIE effect and preparation method of double-BODIPY near-infrared fluorescent dye
A fluorescent dye and near-infrared technology, applied in the direction of organic dyes, luminescent materials, styrene-based dyes, etc., can solve the problems of low yield, small quantity, and many synthesis steps, and achieve high molar extinction coefficient, large Stor Kex shift, the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 compound (II)
[0024] Under anhydrous conditions, terephthalaldehyde (80mg, 0.6mmol) and BODIPY derivative (I) (672mg, 1.4mmol) were dissolved in a mixed solution of methanol and dichloromethane (v / v, 16mL / 8mL), A methanol solution of sodium methoxide (1M, 6 mL) was added dropwise thereto, and after the dropwise addition was completed, the reaction was carried out at room temperature for 6 hours. Add water to quench the reaction, extract with dichloromethane, dry the organic layer over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and separate and purify by silica gel column chromatography, the eluent is dichloromethane-petroleum ether (v:v=9 : 1), to obtain compound (II) (244mg, 38%). 1 H NMR (400MHz, CDCl 3 ): δ7.91(s, 4H), 7.38-7.39(d, J=3.6Hz, 2H), 7.28(s, 2H), 6.97(s, 4H), 6.83-6.84(d, J=3.6Hz, 2H), 6.04(s, 2H), 2.64(s, 6H), 2.60(s, 6H), 2.34(s, 6H), 2.13(s, 12H), 1.44(s, 6H), 1.41(s, 6H ).
Embodiment 2
[0025] Embodiment 2 has the preparation of the double BODIPY class near-infrared fluorescent dye (III) of AIE effect
[0026] Compound (II) (214mg, 0.2mmol), 4-(diphenylamino)benzaldehyde (328mg, 1.2mmol) and freshly dried p-toluenesulfonic acid (17mg, 0.1mmol) were added to 100mL of A two-neck reaction flask equipped with a Dean-Stark device, then add 10mL toluene and 0.2mL piperidine, stir and heat to reflux for 5 hours; after the reaction is completed, cool to room temperature, extract with dichloromethane, wash with water, dry, and evaporate the solvent under reduced pressure Separation and purification by silica gel column chromatography, the eluent is dichloromethane-petroleum ether (v:v=2:3), to obtain double BODIPY near-infrared fluorescent dye (III) with AIE effect (200mg, 48%). 1 H NMR (600MHz, CDCl 3): δ7.91(s, 4H), 7.55-7.63(m, 4H), 7.47-7.48(d, J=8.4Hz, 4H), 7.42(d, J=3.6Hz, 2H), 7.27-7.30( t, J=7.8Hz, 12H), 7.26(s, 4H), 7.23-7.24(d, J=7.8Hz, 8H), 7.13-7.14(d, J...
Embodiment 3
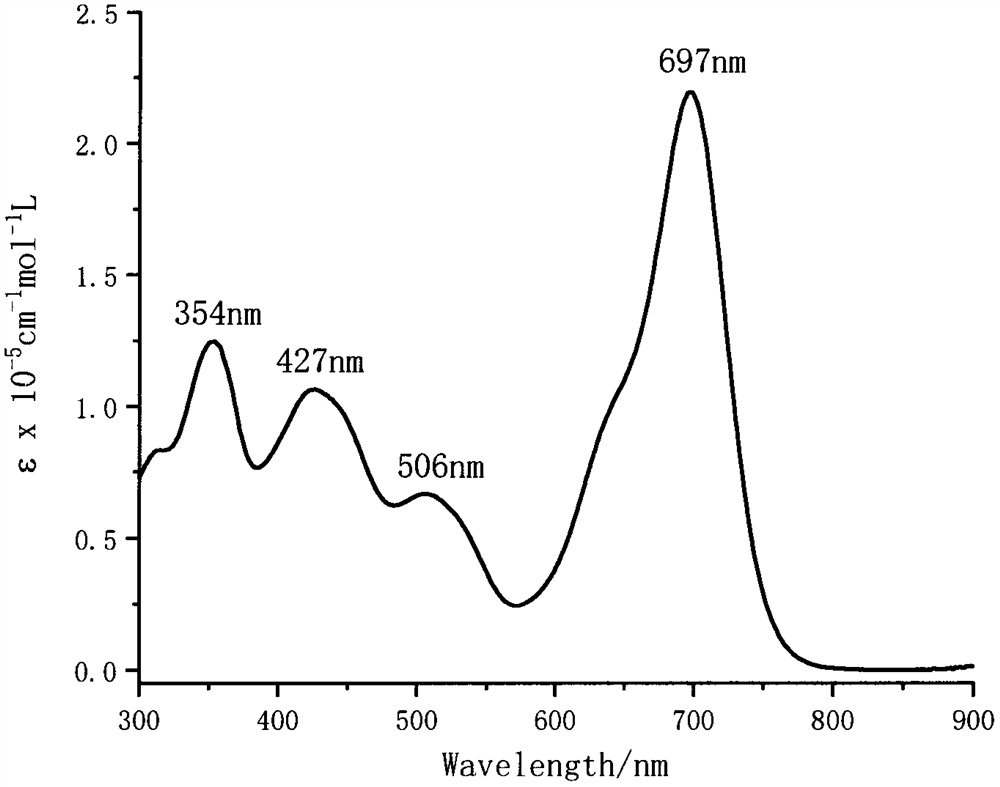
[0027] Embodiment 3 has the double BODIPY class near-infrared fluorescent dye (III) solution ultraviolet-visible absorption spectrum of AIE effect
[0028] Dissolve the double BODIPY type near-infrared fluorescent dye (III) with AIE effect in dichloromethane, and configure the concentration to be 1×10 -5 mol / L dichloromethane solution, and measure its UV-Vis absorption spectrum. figure 1 The ultraviolet-visible absorption spectrum of the fluorescent dye (III) solution prepared for Example 2 of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com