A kind of photoreceptor and its synthesis method
A photoreceptor and synthesis method technology, which is applied in chemical instruments and methods, luminescent materials, fluorescence/phosphorescence, etc., can solve the problems of large cytotoxicity and adverse reactions, narrow excitation spectrum, poor photostability, etc., and achieves easy molecular design, Simple synthesis and the effect of preventing gene mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 (Synthesis of a new photoreceptor):
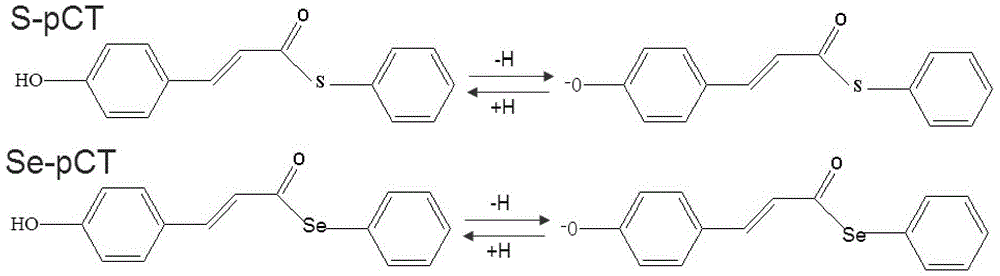
[0019] Such as figure 1 As shown, the optimized structures of the photosensitive flavoprotein chromophore molecules used in the examples are respectively coded S-pCT (neutral molecule) and S-pCT - (Ionic molecule) means that the optimized structure of the chromophore after replacing S atom with selenium atom Se is coded Se-pCT (neutral molecule) and Se-pCT respectively - (Ionic molecule) said.
[0020] Synthesis of sodium phenylselenide: Add 2.40g (0.01mol) magnesium powder to a 250ml three-necked flask, add 35ml anhydrous ether, and start stirring; add 10ml anhydrous ether and 10.7ml bromobenzene mixture to the dropping funnel, The mixed solution in the dropping funnel was slowly added dropwise to the three-necked flask. After the addition was completed, the temperature was slowly raised to 45°C, and the reaction was refluxed for 45 minutes. Stop heating and cool to 36°C. Add 7.01 g of selenium powder to the three-necked flas...
Embodiment 2
[0022] Example 2 (Measurement of absorption spectrum after selenization reaction)
[0023] Weigh a few milligrams to more than ten milligrams of the target product in a small sample tube and add DMSO to make 10 -3 Standard storage solution of M, stored in the refrigerator at -37℃. When using, use H 2 Dilute the O / DMSO buffer to the required concentration and control the DMSO content at 0.5%.
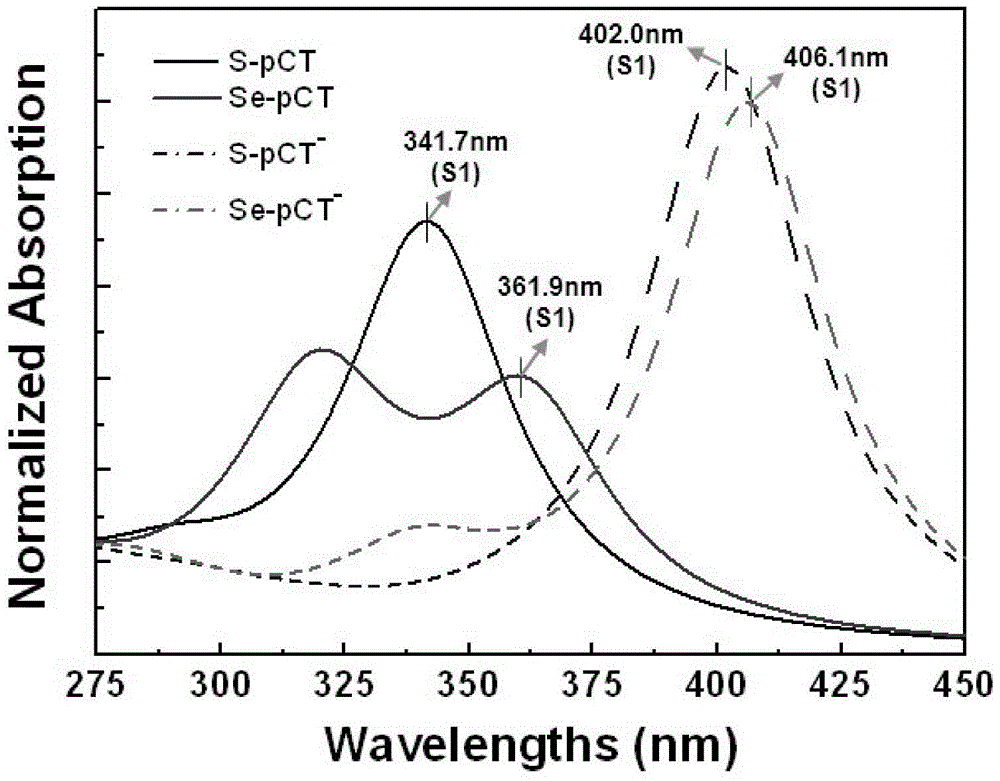
[0024] The absorption spectra of the photosensitive flavoprotein chromophore molecule S-pCT and the target product Se-pCT after selenization were measured in DMSO solution. Take 3μL of stock solution and 3mL of H 2 O / DMSO buffer solution (0.05M Tris-HCl, 50% DMSO, pH=7.5) is prepared into a 5μM test solution, and the test solution is added to a 1cmⅹ1cmⅹ4cm cuvette, and the absorption spectrum and selenium of the sample can be measured separately The change of spectrum after chemical reaction.
[0025] The experimental results of selenization reaction on broadening the red shift of the spectru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com