Method for hydrogen production through methanol reforming by using Pd/ZnFexAl<2-x>O4 catalyst
A technology for reforming hydrogen production and catalysts, which is applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc. It can solve the problems of easy spontaneous combustion and easy deactivation, and achieve Economical cost saving, simple hydrogen production method, and portable hydrogen production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
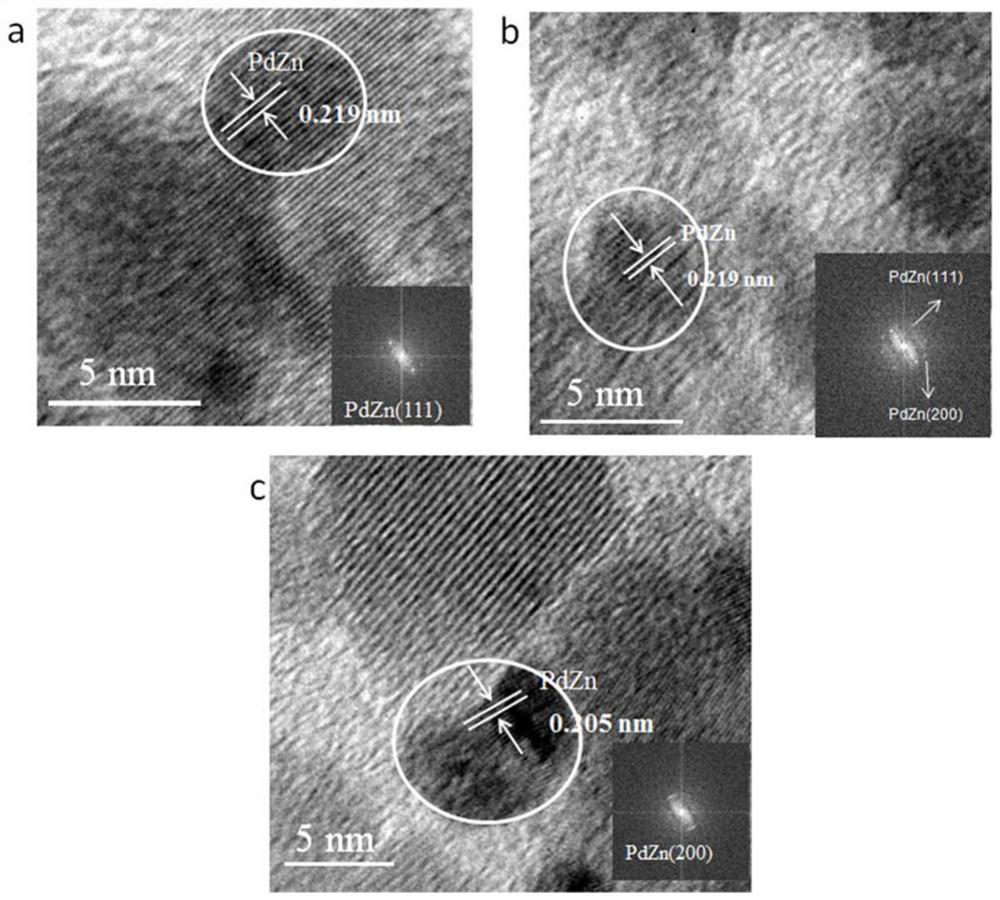
[0047] PdZn / ZnFe with a Pd mass fraction of 0.1 wt.% 0.05 Al 1.95 O 4 The specific method of catalyst catalyzing methanol steam reforming to produce hydrogen is as follows:
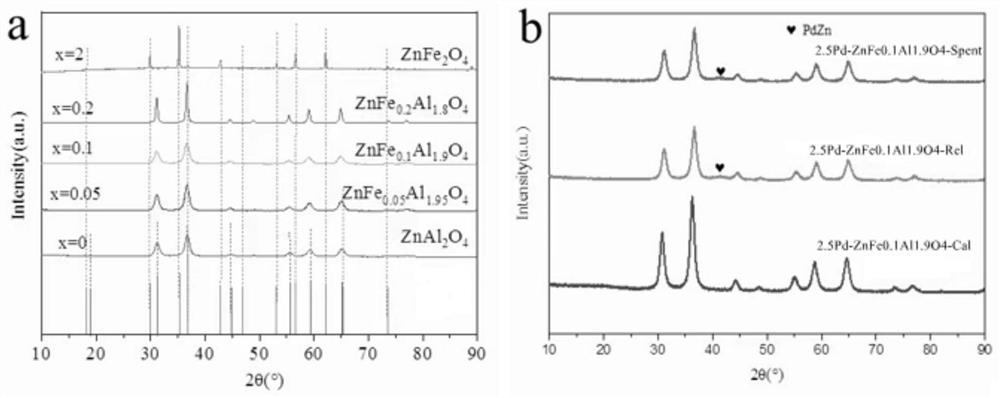
[0048] 1) Add 5.95g of zinc nitrate, 0.4g of ferric nitrate and 13.5g of aluminum trichloride to 100ml of isopropanol, hydrothermally react at 180°C for 10h, and roast the obtained solid at 800°C for 4h to obtain metal iron ion doped ZnFe 0.05 Al 1.95 O 4 Spinel carrier;
[0049] 2) Take 0.0025g Pd(NO 3 ) 2 ·2H 2 The O precursor was placed in a 5 mL sample tube, and was dissolved with a volume ratio of 1:3 nitric acid aqueous solution (0.4 mL) to obtain Pd(NO 3 ) 2 solution; then weigh 1g of ZnFe 0.05 Al 1.95 O 4 The carrier was placed in a 100mL beaker, and it was immersed into Pd(NO) in an equal amount at room temperature. 3 ) 2 In the solution, after filtration, the obtained solid was placed in an oven at 100 °C to dry overnight, and finally the dried solid was calcined at 400 °C for 3 h...
Embodiment 2
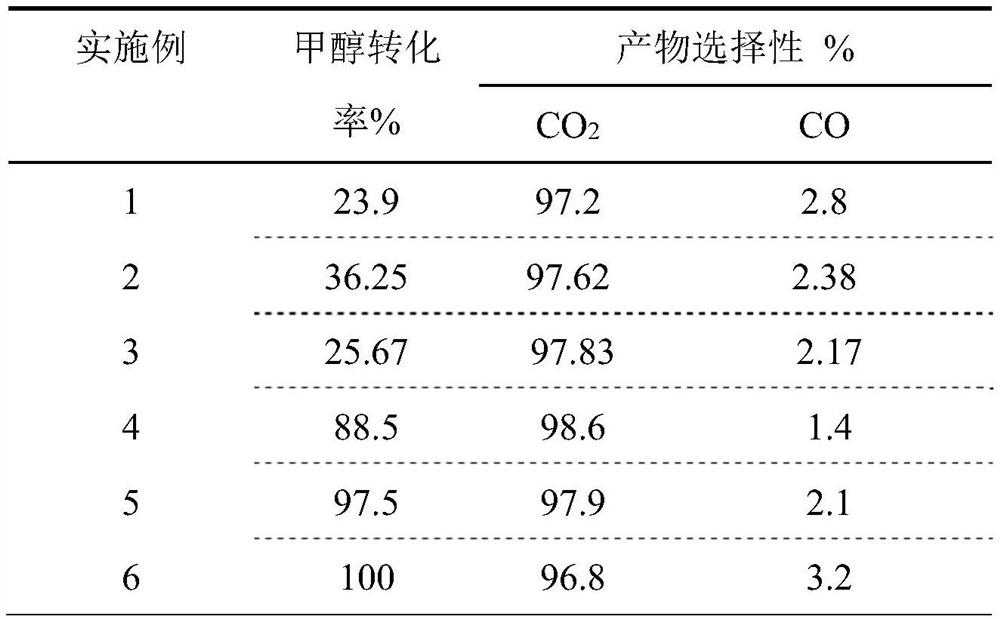
[0053]PdZn / ZnFe with a Pd mass fraction of 0.1 wt.% 0.1 Al 1.9 O 4 The specific method of catalyst catalyzing methanol steam reforming for hydrogen production refers to Example 1, the difference is: the consumption of ferric nitrate in step 1) becomes 0.8g; the remaining steps are the same as in Example 1; the reaction results are shown in Table 1: methanol conversion The yield was 36.25%, while the selectivity to CO by-product was 2.38%.
Embodiment 3
[0055] PdZn / ZnFe with a Pd mass fraction of 0.1 wt.% 0.2 Al 1.8 O 4 The specific method of catalyst catalyzing methanol steam reforming to produce hydrogen is with reference to Example 1, and the difference is: the consumption of ferric nitrate in step 1) becomes 1.6 g; the remaining steps are the same as in Example 1; the reaction results are shown in Table 1: methanol conversion The yield was 25.67%, while the selectivity to CO by-product was 2.17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com