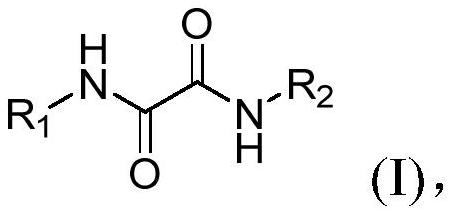
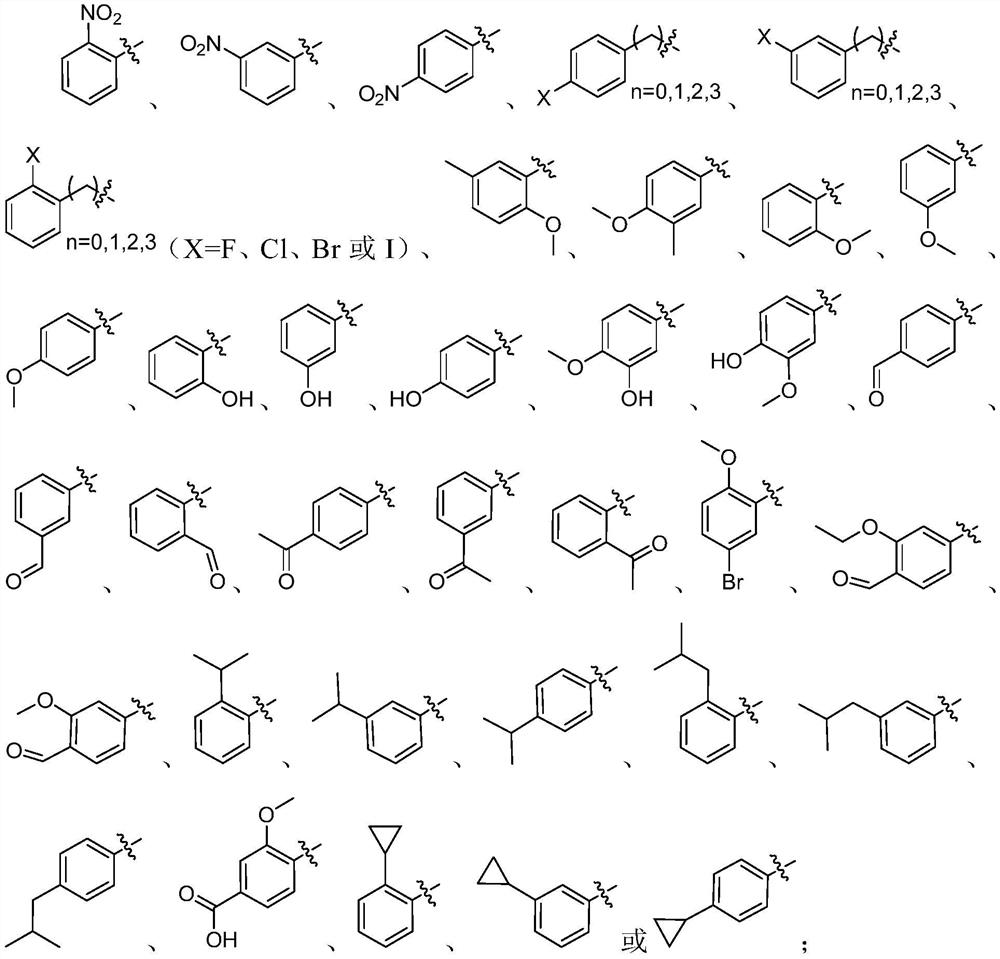
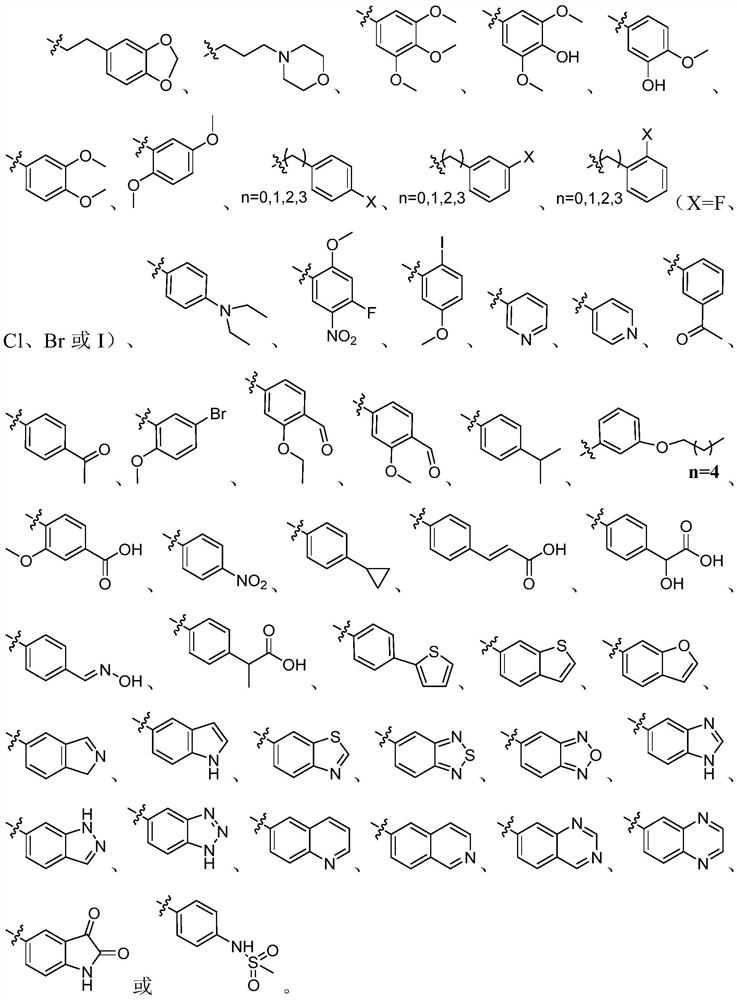
Oxamide neuraminidase inhibitor and preparation method and application thereof
A technology of neuraminidase and oxamide, which is applied in the field of biomedicine, can solve the problems of Tamiflu’s expensive raw materials, serious virus resistance, and complicated synthesis process, and achieve good druggability, low toxicity, and simple molecular structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048]The preparation method of above-mentioned oxamide neuraminidase inhibitor, the equation of preparation method is as follows:
[0049]
[0050] Described preparation method specifically comprises the following steps:
[0051] (1) form a reaction system with substituted aniline, monoethyl oxalyl chloride, triethylamine and ethyl acetate, and obtain the intermediate of formula (II) through after-treatment after the reaction;
[0052] (2) dissolving the intermediate of formula (II) obtained in step (1) in an organic solvent, adding potassium hydroxide to form a reaction system, and obtaining the intermediate of formula (III) after the reaction;
[0053] (3) Dissolve the intermediate of formula (III) obtained in step (2) in an organic solvent, add 1-hydroxybenzotriazole (HOBt) and 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide hydrochloride (EDCl) forms a reaction system, and after the reaction, the oxamide inhibitors shown in the formula (I) are obtained through aftertre...
Embodiment 1
[0084] N 1 -(3-Chlorobenzyl)-N 2 -(3-hydroxyl-4-methoxyphenyl) oxalamide, its structural formula is as shown in formula I:
[0085]
[0086] Concrete synthetic steps are as follows:
[0087] (1) Accurately weigh 2.83g (20mmoL) of 3-chlorobenzylamine in a 150ml round bottom flask, add 4.17mL (30mmoL) of triethylamine and 80.00mL of ethyl acetate, and then use a constant pressure dropping funnel at 0°C Slowly add 3.36mL (30mmoL) monoethyl oxalyl chloride dropwise to the system, and stir the reaction at room temperature 25°C for 6 hours, after the reaction is completed. Add 100mL of distilled water to the reaction system, adjust the pH to 3 with concentrated hydrochloric acid, then add 150mL of ethyl acetate to the reaction solution for extraction and liquid separation, take the organic phase, and then carry out vacuum distillation on the organic phase to obtain the intermediate of formula (II) The crude product of the body was purified by column chromatography to obtain a ...
Embodiment 2
[0094] N 1 -(3-Chlorobenzyl)-N 2 -(3,4,5-trimethoxyphenyl)oxalamide, whose structural formula is as follows, was prepared by a method similar to Example 1.
[0095]
[0096] White solid, 81% yield, IC 50 The value is 0.24±0.11 μM, and the melting point is 215.5-215.7°C.
[0097] 1 H NMR (400MHz, DMSO-d 6 )δ10.55(s,1H),9.59(t,J=6.4Hz,1H),7.42–7.29(m,6H),4.42(d,J=6.4Hz,2H),3.77(s,6H), 3.66(s,3H). 13 C NMR (100MHz, DMSO-d 6 )δ160.75, 158.67, 153.11, 141.66, 134.82, 134.11, 133.46, 130.71, 127.71, 127.43, 126.54, 98.79, 60.58, 56.23, 42.58.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com