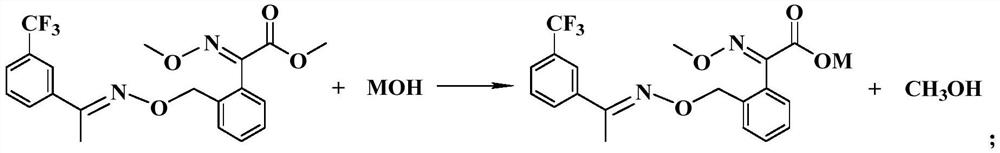
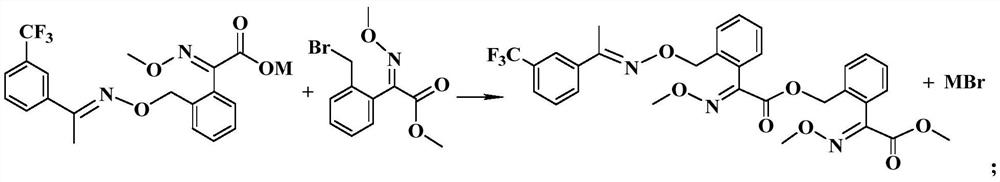
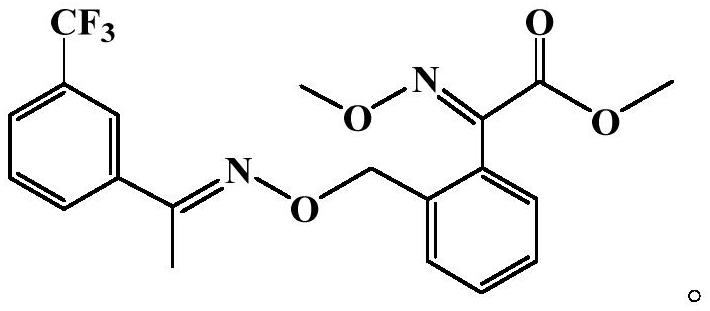
Preparation method of trifloxystrobin characteristic impurity
A technology of trifloxystrobin and impurities, which is applied in the field of organic compound preparation, can solve the problems of low yield of target products, and achieve the effects of good quality control, no side reactions, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] This example provides a characteristic impurity of trifloxystrobin (E)-2-((E)-2-methoxy-1-(methoxyimino)-2-ethoxy)phenyl-2-(methoxy The preparation method of oxyimino)-2-(2-(((E)-1-(3-(trifluoromethyl)phenyl)ethyleneamino)methoxy)phenyl)acetic acid methyl ester, specifically Proceed as follows:
[0053] (1) Synthesis of Sodium Oxygenate
[0054]
[0055] Add 620.1g DMF and 206.9g trifloxystrobin (external standard content is 98%) in reaction kettle, start to stir, then heat up to 30 ℃, slowly add 21.8g sodium hydroxide (96%, 0.525mol), after reacting for 5h After the reaction was detected by HPLC (normalized raw material98%), the reaction solution I was obtained.
[0056] (2) Synthesis of characteristic impurities of trifloxystrobin
[0057]
[0058] Add a total of 174.8g bromoxime ether (90%, 0.55mol) in batches to the reaction solution I obtained in step (1), carry out the condensation reaction at 30°C, take a sample for detection, until the sodium oxalate is...
Embodiment 2
[0064] This example provides a characteristic impurity of trifloxystrobin (E)-2-((E)-2-methoxy-1-(methoxyimino)-2-ethoxy)phenyl-2-(methoxy The preparation method of oxyimino)-2-(2-(((E)-1-(3-(trifluoromethyl)phenyl)ethyleneamino)methoxy)phenyl)acetic acid methyl ester, specifically Proceed as follows:
[0065] (1) Synthesis of Sodium Oxygenate
[0066]
[0067] Add 784.3g DMF and 207.1g trifloxystrobin (external standard content is 98%) in reaction kettle, start to stir, then heat up to 60 ℃, slowly add 20.8g sodium hydroxide (96%, 0.5mol), after reacting for 2h After the reaction was detected by HPLC (normalized raw material98%), the reaction solution I was obtained.
[0068] (2) Synthesis of characteristic impurities of trifloxystrobin
[0069]
[0070] Add a total of 190.9g bromoxime ether (90%, 0.6mol) in batches to the reaction solution I obtained in step (1), carry out the condensation reaction at 60°C, take a sample for detection, until the sodium oxalate is co...
Embodiment 3
[0076] This example provides a characteristic impurity of trifloxystrobin (E)-2-((E)-2-methoxy-1-(methoxyimino)-2-ethoxy)phenyl-2-(methoxy The preparation method of oxyimino)-2-(2-(((E)-1-(3-(trifluoromethyl)phenyl)ethyleneamino)methoxy)phenyl)acetic acid methyl ester, specifically Proceed as follows:
[0077] (1) Synthesis of Sodium Oxygenate
[0078]
[0079] Add 1000.4g DMF and 206.5g trifloxystrobin (external standard content is 98%) in reaction kettle, start to stir, then heat up to 90 ℃, slowly add 25.1g sodium hydroxide (96%, 0.6mol), after reaction 1h After completion of the HPLC detection reaction (normalized raw material98%), the reaction solution I was obtained.
[0080] (2) Synthesis of characteristic impurities of trifloxystrobin
[0081]
[0082] Add a total of 238.8g bromoxime ether (90%, 0.75mol) in batches to the reaction solution I obtained in step (1), carry out condensation reaction at 90°C, take samples for detection, until the sodium oxalate is com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com