Method for preparing 2-chloro-4-(1H-pyrazol-3-yl) benzonitrile by one-step method
A technology of benzonitrile and pyrazole, which is applied in the field of one-step preparation of 2-chloro-4-benzonitrile, can solve the problems of difficulty in chlorination reaction and many solvents, and achieve fast reaction speed, simple method and reduced production cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
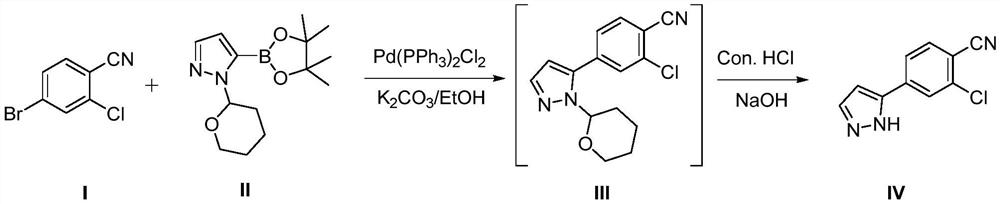
[0030] At room temperature, add compound I (2kg), compound II (3.3kg) and ethanol (5L) into the reaction kettle and stir to dissolve, then add potassium carbonate (3.2kg), vacuumize nitrogen replacement 4 times under stirring, nitrogen Add bis(triphenylphosphine)palladium dichloride (25.4g) under protection, then raise the temperature to 75°C to reflux, stir the reaction overnight (16h), and cool down to room temperature after the reaction is completed, remove the insoluble matter by filtration to obtain compound III in ethanol solution, under the protection of nitrogen, add concentrated hydrochloric acid (1.5kg) to the ethanol solution of the obtained compound III, stir and react overnight at room temperature, add 10% sodium hydroxide solution (5kg) after the reaction to adjust the pH to 6-7, and continue stirring Centrifuged after 30min, rinsed with 50% ethanol-water (1.25L) to obtain the crude wet product of compound IV; the crude wet product was added to ethanol (6.5L) and ...
Embodiment 2
[0032] At room temperature, add compound I (2kg), compound II (3.3kg) and ethanol (5L) into the reaction kettle and stir to dissolve, then add potassium carbonate (3.4kg), vacuumize nitrogen replacement 3 times under stirring, nitrogen Added bis(triphenylphosphine)palladium dichloride (25.4g) under protection, then raised the temperature to 80°C to reflux, stirred and reacted overnight (14h), cooled down to room temperature after the reaction was completed, filtered to remove insoluble matter, and obtained the ethanol solution of compound III , under the protection of nitrogen, add concentrated hydrochloric acid (1.8kg) to the ethanol solution of the obtained compound III, stir and react overnight at room temperature, add 10% sodium hydroxide solution (5.9kg) after the reaction to adjust the pH to 6-7, continue stirring Centrifuged after 30min, rinsed with 50% ethanol-water (1.25L) to obtain the crude wet product of compound IV; the crude wet product was added to ethanol (6.5L)...
Embodiment 3
[0034] At room temperature, add compound I (2kg), compound II (3.3kg) and ethanol (5L) into the reaction kettle and stir to dissolve, then add potassium carbonate (3.2kg), vacuumize nitrogen replacement twice under stirring, nitrogen Add bis(triphenylphosphine)palladium dichloride (25.4g) under protection, then raise the temperature to reflux at 85°C, stir the reaction overnight (12h), cool down to room temperature after the reaction is completed, remove the insoluble matter by filtration, and obtain the ethanol solution of compound III , under the protection of nitrogen, add concentrated hydrochloric acid (2.1kg) to the ethanol solution of the obtained compound III, stir and react overnight at room temperature, add 10% sodium hydroxide solution (7.0kg) after the reaction to adjust the pH to 6-7, and continue stirring Centrifuge after 30min, rinse with 50% ethanol-water (1.25L) to obtain the crude wet product of Compound IV; add the obtained crude wet product to ethanol (6.5L) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com