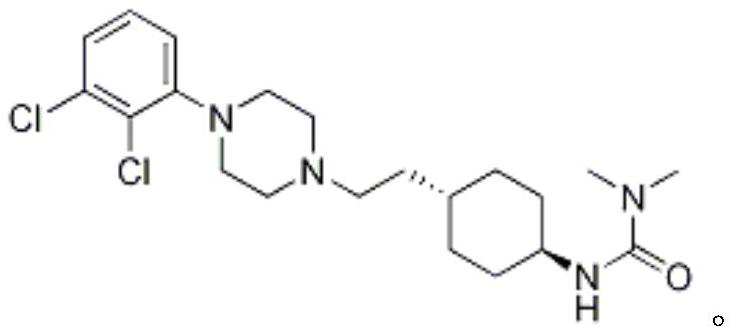
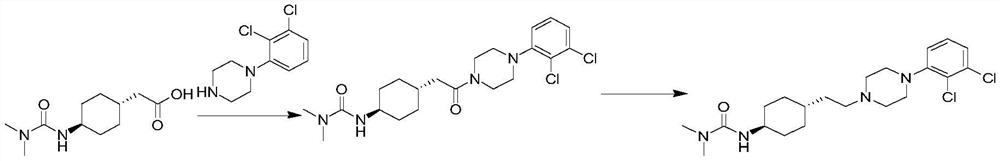
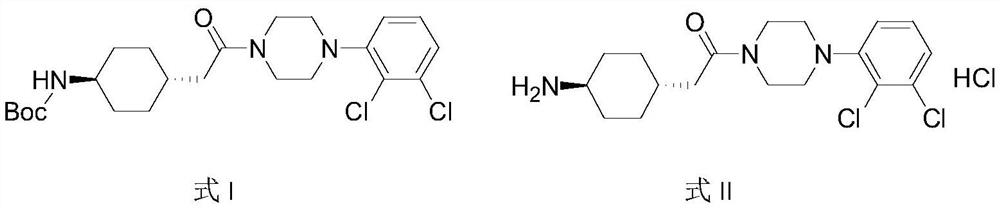
Preparation method of cariprazine
A technology of piperazine and compounds, which is applied in the field of preparation of cariprazine, can solve the problems of long reaction time, complex process of cariprazine intermediates, low yield and purity, and achieve short reaction time, high purity, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: (4-{2-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-2-oxoethyl}-cyclohexyl)-tert-butyl carbamate preparation of
[0039] Starting material 150.1g (1.0eq) 4-tert-butoxycarbonylamino-cyclohexyl)-acetic acid (SM01), 187.3g (1.2eq) 1-(2,3-dichlorophenyl)piperazine (SM02) , 134.3g (1.2eq) EDCI and 1L dichloromethane were added to a 2L reaction flask, and 202mL (2.3eq) triethylamine was added under stirring, and the temperature was raised to 40°C for reflux reaction. After 2h, the reaction was completed, and stirred overnight at room temperature; Cleared, stirred and separated, the organic phase was concentrated, the crude product was refluxed with methanol for beating, filtered, washed with methanol, and dried to finally obtain 183.70g (4-{2-[4-(2,3-dichloro-phenyl)-piperazine -1-yl]-2-oxoethyl}-cyclohexyl)-tert-butyl carbamate (IM01), yield 66.9%, purity 98.5%.
Embodiment 2
[0040] Example 2: (4-{2-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-2-oxoethyl}-cyclohexyl)-tert-butyl carbamate preparation of
[0041]
[0042] Dissolve the starting material 0.20kg (1.0eq) 4-tert-butoxycarbonylamino-cyclohexyl)-acetic acid (SM01) in tetrahydrofuran, then add 0.18kg (1.2eq) EDCI, 0.13kg (1.2eq) HOBT and 0.24kg (3.0eq) triethylamine, stirred at room temperature for about 1 hour, after the reaction was completed, add 0.25kg (1.2eq) 1-(2,3-dichlorophenyl)piperazine (SM02), and then heated to 50-60 ℃ for the reaction, and the reaction ended after 4 hours; after the reaction was completed, 3 times the volume of tetrahydrofuran was added for crystallization, and then the crude product was obtained by filtration to obtain (4-{2-[4-(2,3-dichloro-phenyl) -piperazin-1-yl]-2-oxoethyl}-cyclohexyl)-tert-butyl carbamate (IM01). Yield 93.90%, purity 99.65%.
[0043] NMR: 1 H NMR (300MHz, CDCl 3 ):δ=7.21-7.13(m,2H),6.93-6.90(m,1H),4.38(s,1H),3.80(s,2H),3.66-3.63(m,2H),...
Embodiment 3
[0044] Example 3: Trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexylamine hydrochloride preparation
[0045]
[0046] Add 0.34kg (1.0eq) of IM01 to 1.37kg of acetonitrile, then add 0.2kg (3.0eq) of 37% concentrated hydrochloric acid, heat up to 60-65°C to react, react for 3-4h, after the reaction is complete, cool down, filter, the amount of acetonitrile Rinse the filter cake and dry to obtain trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexylamine Hydrochloride (IM02), yield 98.85%, purity 99.86%.
[0047] NMR: 1 H NMR(300MHz,d-DMSO):δ=8.1370(s,3H),7.1239-7.0817(d,2H),6.9022-6.8705(t.1H),3.68401(s,2H),3.5653(s,2H) ,2.9258-2.8468(m,5H),2.1947-2.1728(d,2H),2.0095-1.9679(d,2H,),1.8355-1.7916(d,3H),1.4355-1.3200(m,2H),1.0471-0.9174 (m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com