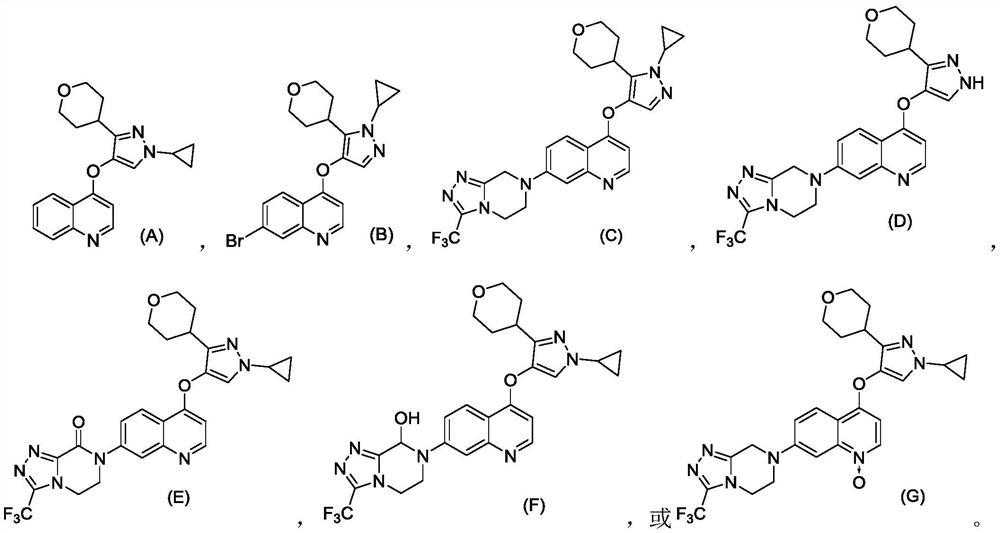
Quinoline compound as well as preparation method and application thereof
A compound, quinoline technology, applied in the field of medicinal chemistry, can solve the problems of off-target toxicity and side effects, low target selectivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 4-((1-cyclopropyl-3-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-yl)oxy)-7-(3-(trifluoro Preparation of methyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)quinoline
[0048]
[0049] Step 1: Preparation of 2-bromo-1-(tetrahydro-2H-pyran-4-yl)ethan-1-one
[0050]
[0051]Under nitrogen protection, add methanol (100mL) and 1-(tetrahydro-2H-pyran-4-yl)ethanone (20.0g, 156mmol) in sequence to a 1000mL three-necked flask, cool down to below -15°C, and slowly drop Enter liquid bromine and keep the temperature below -15°C. After dropping, raise the temperature to 0°C, react for 45 minutes, then raise the temperature to 10°C, react for 45 minutes, keep the internal temperature below room temperature and slowly add 11mol / L sulfuric acid (55mL) dropwise, and react overnight at room temperature. Monitor the completion of the reaction, add ethyl acetate and sodium chloride aqueous solution for extraction, combine the organic layers, adjust the pH value of the o...
Embodiment 2
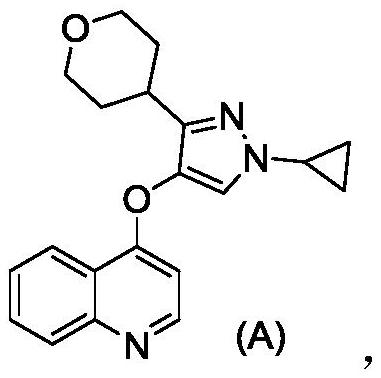
[0086] Example 2: 4-((1-cyclopropyl-3-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-yl)oxy)quinoline
[0087]
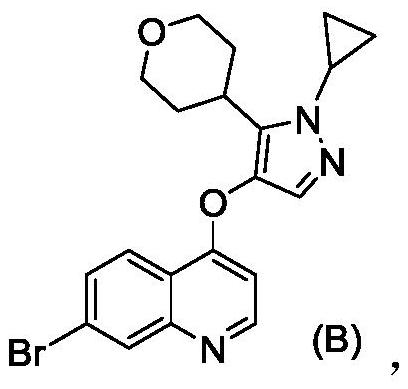
[0088] 7-bromo-4-((1-cyclopropyl-3-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-yl)oxy)quinoline (1.00g, 2.41 mmol), Pd / C (0.20g) were dissolved in methanol (20mL), replaced by hydrogen, and kept stirring at room temperature under hydrogen atmosphere for 4-5hrs. LC-MS monitored that the reaction was complete, filtered, and concentrated the mother liquor to dryness to obtain the title compound, a total of 0.92g . ESI-MS[M+H] + m / z:336.2, 1 H NMR (500MHz, DMSO-d 6 )δ9.14-9.15(1H, J=6.5Hz,d),8.55-8.57(1H,J=6.5Hz,dd),8.30-8.32(1H,J=8.5Hz,d),8.20-8.23(1H ,J=8Hz,t),8.10(1H,s),7.98-8.02(1H,J 1 =8Hz,t),7.29-7.30(1H,J=6.5Hz,d),3.74-3.75(1H,m),3.25-3.30;3.76-3.80(4H,m),2.78-2.84(1H,m) ,1.62-1.71(4H,m),0.97-1.11(4H,m).
Embodiment 3
[0089] Example 3: 4-((1-cyclopropyl-5-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-yl)oxy)-7-(3-(tri Fluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)quinoline
[0090]
[0091] Step 1: Preparation of 1-cyclopropyl-5-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-ol
[0092]
[0093] The reaction mother liquor of 1-cyclopropyl-3-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-ol in Step 6 of Example 1 was subjected to column chromatography to obtain 15 g of crude product. Take 10 g of the crude product and add ethyl acetate (50 mL), heat up and reflux to dissolve, slowly add petroleum ether (50 mL), turn off heating and cooling to crystallize to 40°C, heat filter, and dry the filter cake under vacuum for 3 hours to obtain the title compound, the yield is 35.0 %. ESI-MS[M+H] + m / z:209.1, 1 H NMR (500MHz, DMSO-d 6 )δ7.97(1H,s),6.85(1H,s),3.43-3.46; 3.92-3.95(4H,m),3.36-3.41(1H,m),3.11-3.18(1H,m),1.56- 1.59; 2.02-2.10(4H,m),0.92-0.97(4H,m).
[0094] Step 2: Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com