Hemi-cucurbituril C33H39O3N9 containing aniline structure and synthesis method thereof
A technology of C33H39O3N9 and C33H39O3N, which is applied in the field of semimelon ring C33H39O3N9 and its synthesis, to achieve the effect of easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
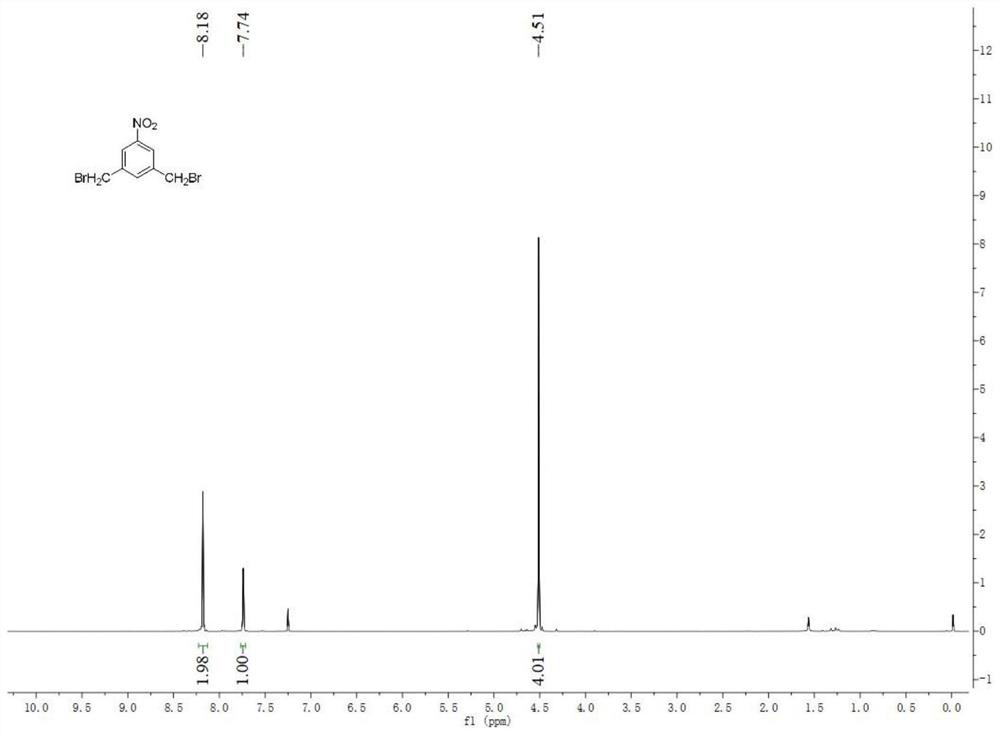
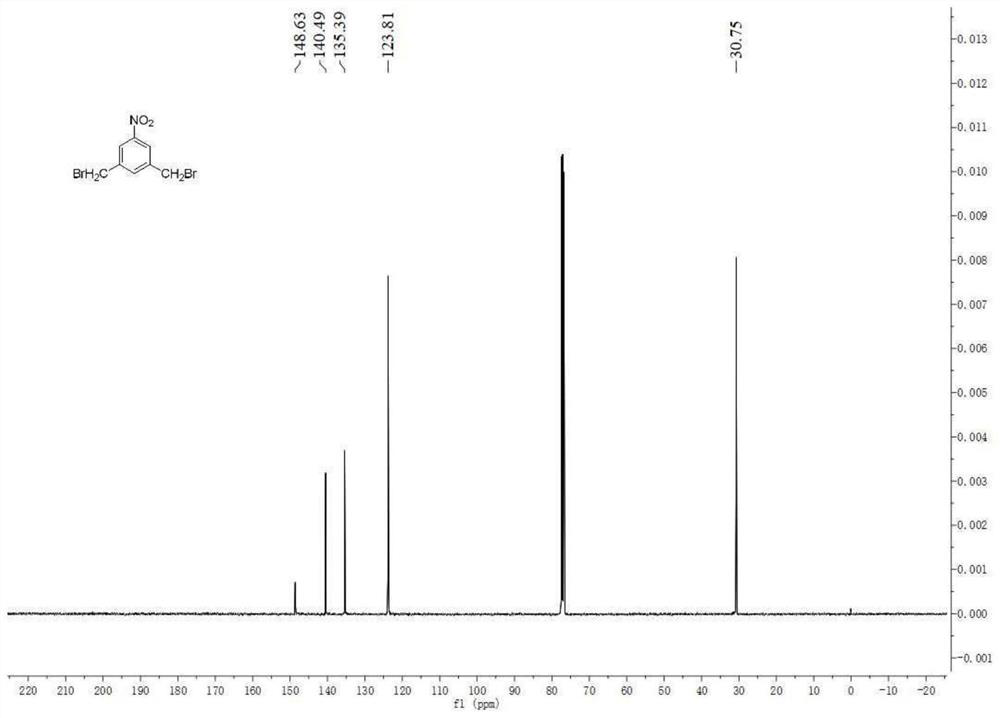
[0055] Embodiment 1: Synthesis of intermediate 3,5 bis-(bromomethyl)nitrobenzene (3):
[0056] The compound 5-nitrobenzene-1,3-dioic acid (1) (59.7mmol, 12.66g, 1eq) was weighed and dissolved in THF (100mL), stirred until completely dissolved, and the solution was cooled to 0°C. NaBH 4 (208.95mmol, 7.94g, 3.5eq) was added to the solution in batches, and when the solution stopped bubbling, slowly added BF 3 ·Et 2 O (0.208mol, 28.4mL, 3.5eq) was added dropwise to the reaction liquid within 1h, the temperature was allowed to rise to room temperature naturally, and the reaction was kept at this temperature for more than 16h, after the reaction was completed. The reaction solution was cooled to 0° C., and the reaction solution was carefully quenched with aqueous sodium hydroxide (1 mol / L, 200 mL), and the reaction solution was stirred for 3 h to be completely quenched. Part of the solvent was spun off from the reaction solution with a rotary evaporator. The suspension was extra...
Embodiment 2
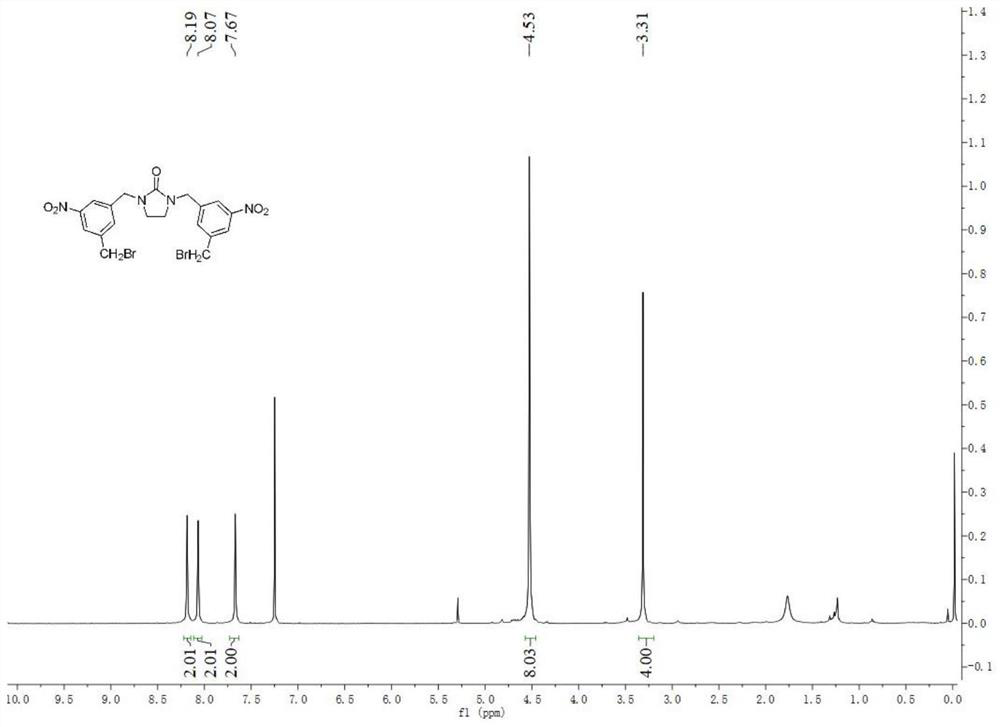
[0057] Example 2: Synthesis of intermediate 3,5-bis((methylene)(imidazolidin-2-one)-5-nitrobenzene) (4):
[0058] Hemiglycoside (174mmol, 15g, 10.77eq) was weighed and dissolved in DMF (45mL), compound 3 (16.2mmol, 5g, 1eq) was added to the reaction solution, and stirred overnight at 100°C. Spot the plate to monitor the reaction. After the reaction is complete, spin the DMF to dryness, pour the suspension into 100mL water, extract the aqueous phase with dichloromethane three times or more, combine the organic phases, wash with water and brine, and wash with Na 2 SO 4 dry. Filter and spin dry to obtain a crude oily liquid. The crude product was purified by silica gel column chromatography (EtOAc:CH 3 OH=10:1) to obtain a white solid product with a yield of 25.1%.
Embodiment 3
[0059] Example 3: Synthesis of intermediate 3,5-bis((methylene)(imidazolidin-2-one)-5-nitrobenzene) (4):
[0060] Weigh hemiglycoside (174mmol, 15g, 10.77eq), tetrabutylammonium bromide (0.387mmol, 0.13g, 0.3eq), sodium iodide (0.52mmol, 0.08g, 0.4eq) dissolved in DMF (45mL) , compound 3 (16.2mmol, 5g, 1eq) was added to the reaction solution and stirred overnight at 100°C. Spot the plate to monitor the reaction. After the reaction is complete, spin the DMF to dryness, pour the suspension into 100mL water, extract the aqueous phase with dichloromethane three times or more, combine the organic phases, wash with water and brine, and wash with Na 2 SO 4 dry. Filter and spin dry to obtain a crude oily liquid. The crude product was purified by silica gel column chromatography (EtOAc:CH 3 OH=10:1) to obtain a white solid product with a yield of 24.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com