Dabrafenib transdermal preparation composition and preparation method of dabrafenib transdermal preparation
A technology for dabrafenib transdermal and transdermal preparations is applied in the field of preparation of dabrafenib transdermal preparation compositions and dabrafenib transdermal preparations, and can solve gastrointestinal irritation, poor specificity and low efficiency and other problems, to achieve the effect of high transdermal absorption rate and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
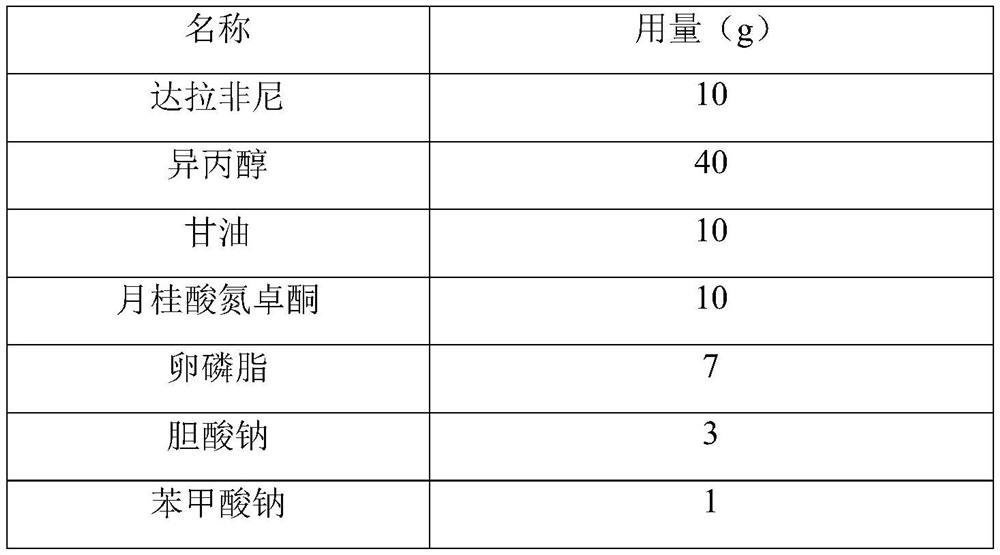
[0028] Table 1 Prescription
[0029]
[0030]
[0031] making process:
[0032] (1) Dissolve 10 g of dabrafenib in 40 g of isopropanol.
[0033] (2) Heat 10g of glycerin, 10g of azone laurate, 7g of lecithin, 3g of sodium cholate, 1g of sodium benzoate, and 20g of stearin into a solution state, stir for 10 minutes using a high-speed homogenizer, and add isopropanol solution and continue stirring for 10 minutes.
[0034] (3) After static cooling, put it into the prepared composite hose and seal it. That is, dabrafenib cream.
[0035] Test results:
[0036] The transdermal absorption diffusion cell was used to detect the transmittance, and the results are shown in Table 2.
[0037] Table 2 Transmittance test results
[0038] time (hours) Dabrafenib Concentration (mg / mL) 3 0.015 6 0.033 12 0.043
Embodiment 2
[0040] Without adding lecithin, the more commonly used transdermal penetration enhancers azone laurate and oleic acid were used to prepare samples, and relevant transdermal tests were carried out.
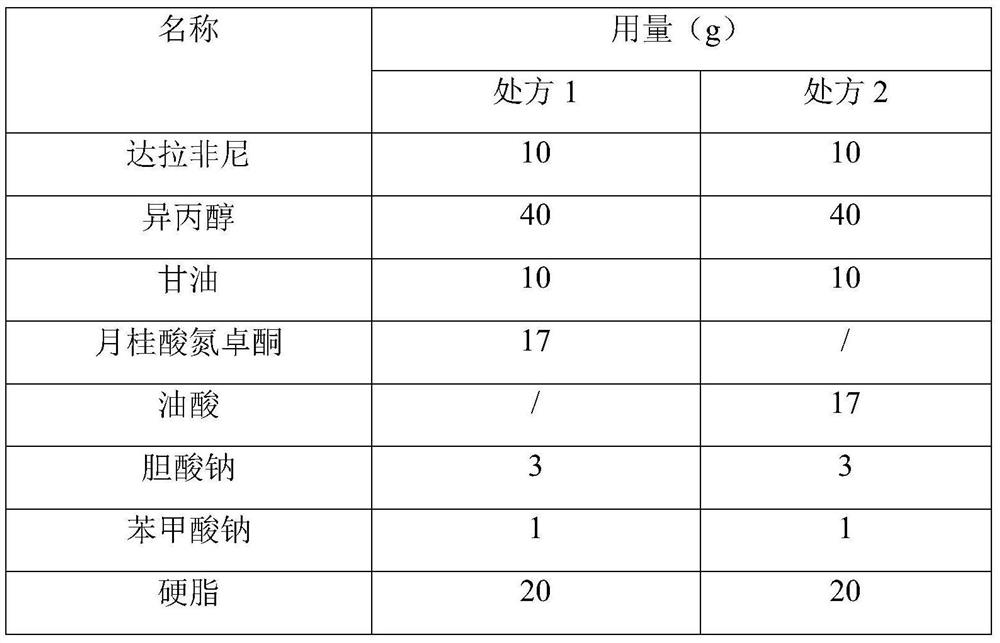
[0041] Table 3 Prescription
[0042]
[0043] making process:
[0044] (1) Dissolve 10 g of dabrafenib in 40 g of isopropanol.
[0045] (2) Heat 10g of glycerin, 17g of azone or oleic acid, 3g of sodium cholate, 1g of sodium benzoate, and 20g of stearin into a solution state, use a high-speed homogenizer to stir for 10 minutes, and add isopropanol containing dabrafenib solution, stirring was continued for 10 minutes.
[0046] (3) After static cooling, put it into the prepared composite hose and seal it. That is, dabrafenib cream.
[0047] Test results:
[0048] The transdermal absorption diffusion cell was used to detect the transmittance, and the test results are shown in Table 4.
[0049] Table 4 Transmittance test results
[0050]
[0051] Conclusion: Without adding...
Embodiment 3
[0053] The test was carried out with azone laurate or lecithin, respectively.
[0054] Table 5 Prescription
[0055]
[0056] making process:
[0057] (1) Dissolve 10 g of dabrafenib in 40 g of isopropanol.
[0058] (2) Heat 10g of glycerin, 17g of azone laurate or lecithin, 10g of oleic acid, 3g of sodium cholate, 1g of sodium benzoate, and 20g of stearin into a solution state, stir for 10 minutes using a high-speed homogenizer, and add The isopropanol solution of Rafenib was stirred for an additional 10 minutes.
[0059] (3) After static cooling, put it into the prepared composite hose and seal it. That is, dabrafenib cream.
[0060] Test results:
[0061] The transdermal absorption diffusion cell was used to detect the transmittance, and the test results are shown in Table 6.
[0062] Table 6 Transmittance test results
[0063]
[0064] Conclusion: The penetration rate of dabrafenib is also significantly lower when acetrene laurate lecithin is used alone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com