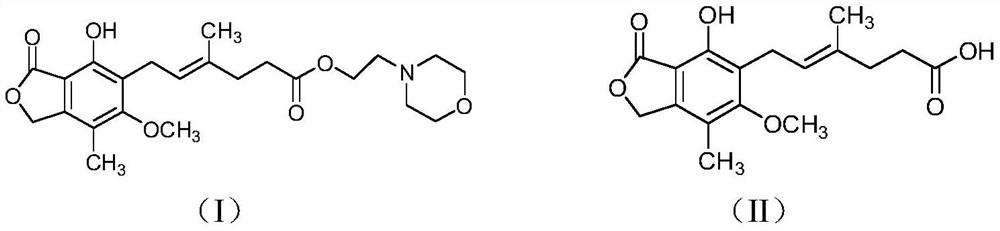
Preparation method of mycophenolate mofetil impurity A
A technology of mycophenolate mofetil and impurities, applied in the field of preparation of mycophenolate mofetil impurity A, to achieve the effects of good reaction selectivity, high reaction yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
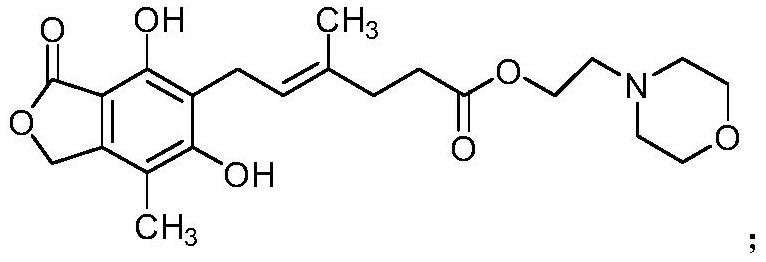
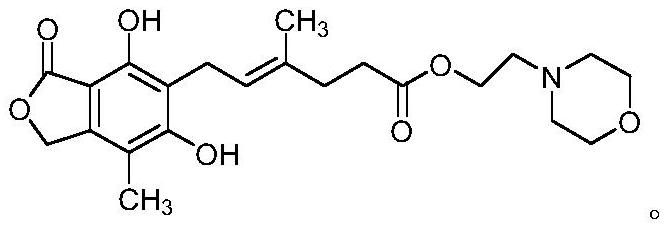
[0031] Under nitrogen protection, add 43.35g of mycophenolate mofetil and 5.56g of magnesium iodide into a three-necked flask, add 433.5ml of dichloromethane, stir and cool down to -15~-10°C, and dropwise add 100.20g of tribromo 200ml of dichloromethane solution of boron chloride (mass-to-mass ratio 1:2), after the dropwise addition is completed, the temperature is slowly raised to 30-35°C, and the reaction is kept for 3-4 hours. After the reaction was completed, under stirring, the reaction mixture was added to 1500ml of ice-water mixture, and the layers were separated. The organic layer was washed with 200ml of 5% aqueous sodium bicarbonate solution, 200ml of saturated brine, dried over anhydrous sodium sulfate for 4 hours, and evaporated under reduced pressure. Dry, obtain the crude product of 37.8g mycophenolate mofetil impurity A.
[0032] Under nitrogen protection, add 37.8g of mycophenolate mofetil impurity A crude product into a three-necked flask, add 380ml of a mixtu...
Embodiment 2
[0034] Under the protection of nitrogen, add 43.35g of mycophenolate mofetil and 2.78g of magnesium iodide into a three-necked flask, add 520ml of dichloromethane, stir and cool down to -15~-10°C, and dropwise add 75.15g of tribromide Boron dichloromethane solution 150ml (mass-to-mass ratio 1:2), after the dropwise addition, slowly raise the temperature to 30-35°C, and keep it warm for 3-4 hours. After the reaction was completed, under stirring, the reaction mixture was added to 1500ml of ice-water mixture, and the layers were separated. The organic layer was washed with 200ml of 5% aqueous sodium bicarbonate solution, 200ml of saturated brine, dried over anhydrous sodium sulfate for 4 hours, and evaporated under reduced pressure. Dry, obtain the crude product of 35.7g mycophenolate mofetil impurity A.
[0035]Under nitrogen protection, add 35.7g of mycophenolate mofetil impurity A crude product into a three-necked flask, add 360ml of a mixture of butyl acetate and ethanol (vo...
Embodiment 3
[0037] Under nitrogen protection, add 43.35g of mycophenolate mofetil and 8.34g of magnesium iodide into a three-necked flask, add 650ml of dichloromethane, stir and cool down to -15~-10°C, and dropwise add 125.26g of tribromide Boron dichloromethane solution 250ml (mass-to-mass ratio 1:2), after the dropwise addition, slowly raise the temperature to 30-35°C, and keep it warm for 3-4 hours. After the reaction was completed, under stirring, the reaction mixture was added to 1500ml of ice-water mixture, and the layers were separated. The organic layer was washed with 200ml of 5% aqueous sodium bicarbonate solution, 200ml of saturated brine, dried over anhydrous sodium sulfate for 4 hours, and evaporated under reduced pressure. Dry, obtain the crude product of 35.9g mycophenolate mofetil impurity A.
[0038] Under nitrogen protection, add 35.9g of mycophenolate mofetil impurity A crude product into a three-necked flask, add 360ml of a mixture of butyl acetate and ethanol (volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com