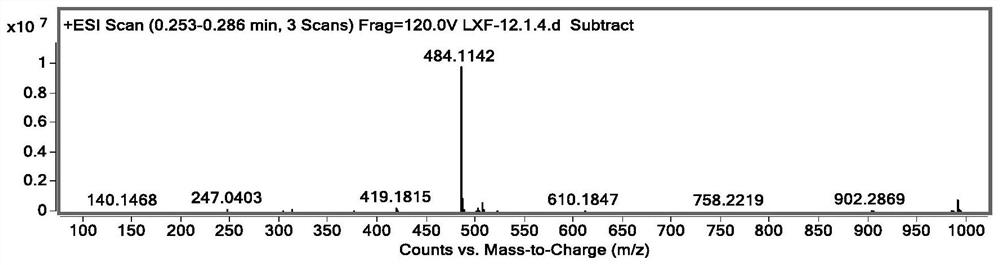
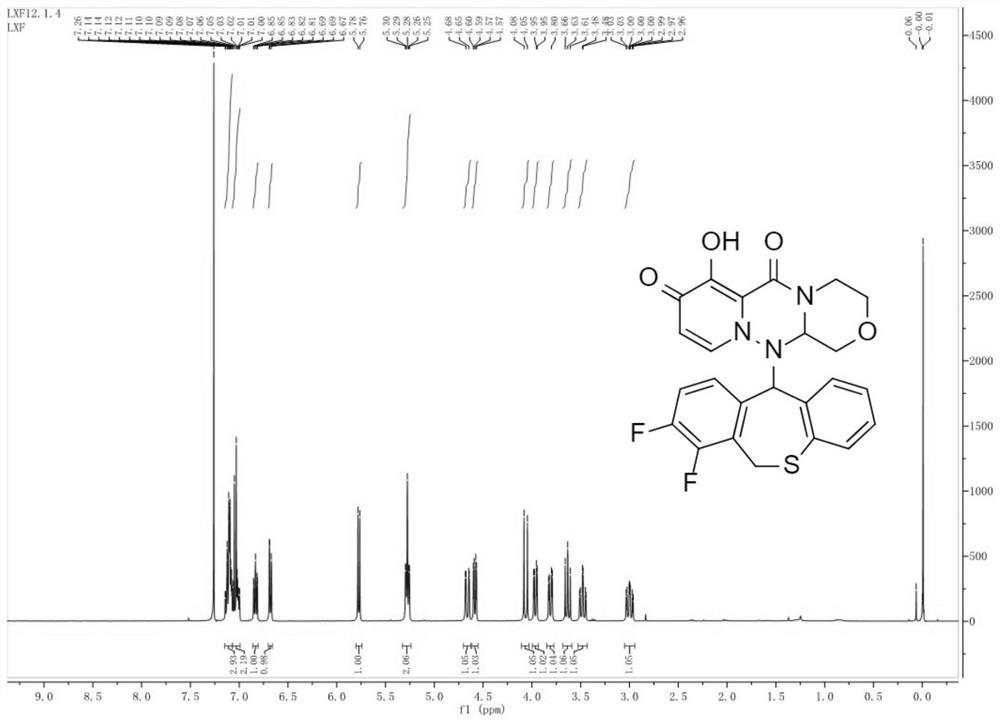
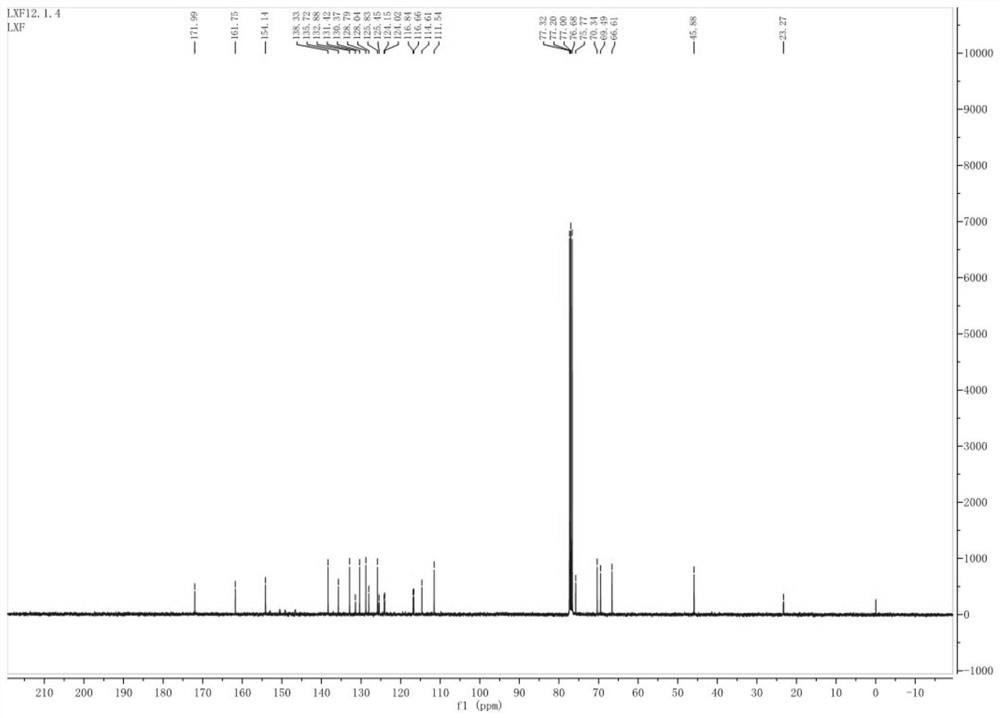
Preparation method of key intermediate of baloxavir marboxil
An intermediate and key technology, which is applied in the field of preparation of key intermediates of baloxavir, can solve problems such as low yield and long reaction time, and achieve the effects of convenient use, environmental protection, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the key intermediate of baloxavir provided by the invention comprises:
[0038]
[0039] S1. Add compound 1 with the structure of formula 1 and compound 2 with the structure of formula 2 into the microwave reactor, under the action of sulfonic acid resin type solid acid catalyst and condensation agent, generate intermediate with the structure of formula 3 in the first solvent Body 3;
[0040] S2. Add intermediate 3 and lithium chloride to the microwave reactor to generate the key intermediate of baloxavir with the structure of formula 4 in the second solvent.
[0041] Wherein, the mass ratio of the sulfonic acid resin type solid acid catalyst to the compound 1 is 3-10:100. For example, it can be 3:100, 5:100, 7:100, 10:100, etc.
[0042] The condensation agent is 1-propyl phosphoric anhydride.
[0043] The molar ratio of condensing agent to compound 1 is 1.5-2.5:1. For example, it can be 1.5:1, 1.8:1, 2:1, 2.4:1, 2.5:1, etc.
[0044] Th...
Embodiment 1
[0055] A preparation method of the key intermediate of baloxavir having the structure shown in formula 4, comprising:
[0056]
[0057] Compound 1 (2.64g, 10mmol), compound 2 (3.27g, 10mmol) and solid acid catalyst HND-580 (0.132g) were added to a 100mL microwave reactor, and 12mL of 50wt% 1-propylphosphoric anhydride was added (12mL solution contains 20.18mmol of 1-propyl phosphoric anhydride); put the reaction kettle into a microwave reactor (XH-800SP Nanocube multifunctional microwave hydrothermal parallel synthesizer), and set the reaction temperature to 150°C , the maximum power is 300w, the heating time is 50min, and the reaction time is 30min. After setting, click the start button and start stirring; after the reaction is over, pour the reaction solution into ice water, filter and recover the catalyst; the filtrate is extracted with dichloromethane, The organic phases were combined, washed successively with saturated aqueous sodium bicarbonate solution and saturated ...
Embodiment 2
[0078] A preparation method of the key intermediate of baloxavir having the structure shown in formula 4, comprising:
[0079] Compound 1 (2.64g, 10mmol), compound 2 (3.27g, 10mmol) and solid acid catalyst HND-586 (0.132g) were added to a 100mL microwave reactor, and 12mL of 50wt% 1-propylphosphoric anhydride was added the ethyl acetate solution; put the reaction kettle into the microwave reactor (XH-800SP Nanocube multifunctional microwave hydrothermal parallel synthesizer), set the reaction temperature to 150°C, the maximum power to 300w, the heating time to 50min, and the reaction time After the setting is completed, click the start button and start stirring; after the reaction is completed, pour the reaction solution into ice water, filter and recover the catalyst; Wash with sodium chloride solution and dry over anhydrous sodium sulfate to obtain a yellow oily compound, which is the crude product of compound 3; add the crude product of compound 3 to a 100mL microwave react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com