Application of lithocholic acid compound in preparation of antibacterial product
An antibacterial product, technology of lithocholic acid, applied in the direction of cosmetic preparations, cosmetics, antibacterial drugs, etc., can solve the problems such as the report of the application of antibacterial products of lithocholic acid and its derivatives.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In this case, a series of lithocholic acid compounds (abbreviated as LA series compounds) were tested for MIC by using the agar double dilution method to investigate the effect of lithocholic acid compounds on methicillin-resistant Staphylococcus aureus (MRSA), methicillin Sensitive Staphylococcus aureus (MSSA), Escherichia coli Esbls+, Escherichia coli Esbls-, the in vitro antibacterial activity (MIC value) of Pseudomonas aeruginosa, and compare with positive control levofloxacin; Wherein, MIC 50 and MIC 90 Respectively refer to the MIC (MIC: Minimum Inhibitory Concentration) required to inhibit 50% or 90% of the tested bacteria.
[0028] 1.1 Test product
[0029] Table 1 Information about the test product
[0030]
[0031] The structural formula of above-mentioned LA series compound is as follows:
[0032]
[0033] The information of the test items is shown in Table 1. The above test items were all prepared with DMSO to a maximum concentration of 3.84 mg / ml b...
Embodiment 2
[0087] On the basis of Example 1, this example further carried out the MIC test of compound LA-1 and compound LA-9 to methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA) ( Expand the number of strains to 20).
[0088] 1 Test product and reference product
[0089] 1.1 Test product
[0090] Table 5 information on the test product
[0091]
[0092] The information of the test products is shown in Table 5. The above test products were all prepared with DMSO to a maximum concentration of 3.84mg / ml before use, and then double-diluted sequentially.
[0093] 1.2 Reference substance
[0094] Name: Levofloxacin Lactate Sodium Chloride Injection, commercially available.
[0095] Batch number: 118190906 Manufacturer: Zhejiang Pharmaceutical Co., Ltd.
[0096] Properties: Pale yellow liquid Specification: 500mg / 250ml
[0097] Storage Conditions: Sealed in a cool, dry place shading.
[0098] 2 Test instruments and reagents
[009...
Embodiment 3
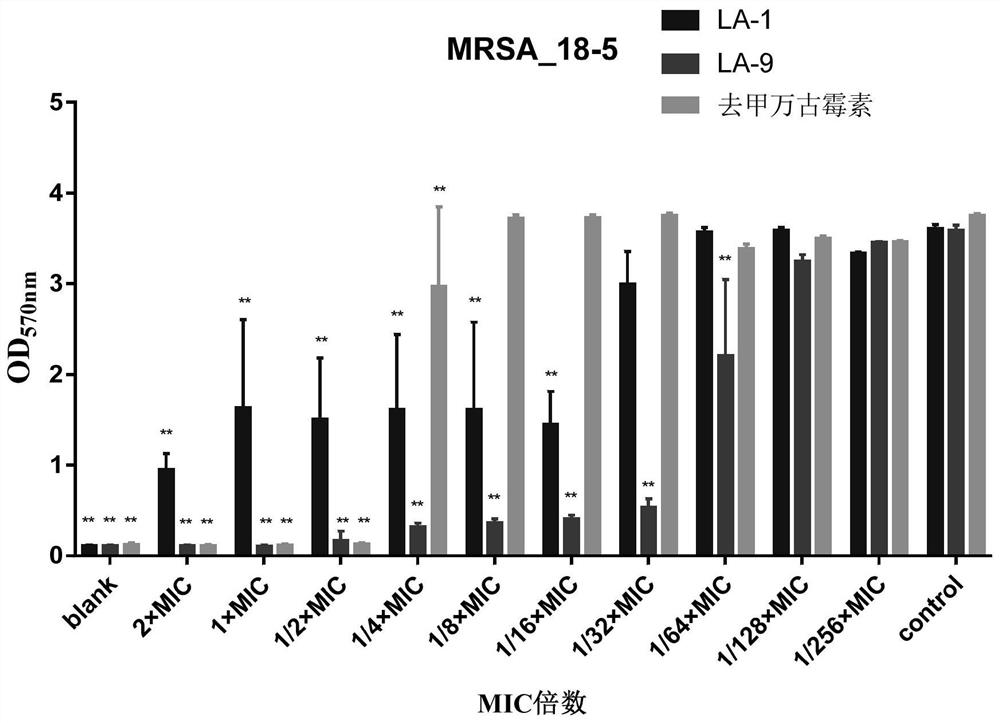
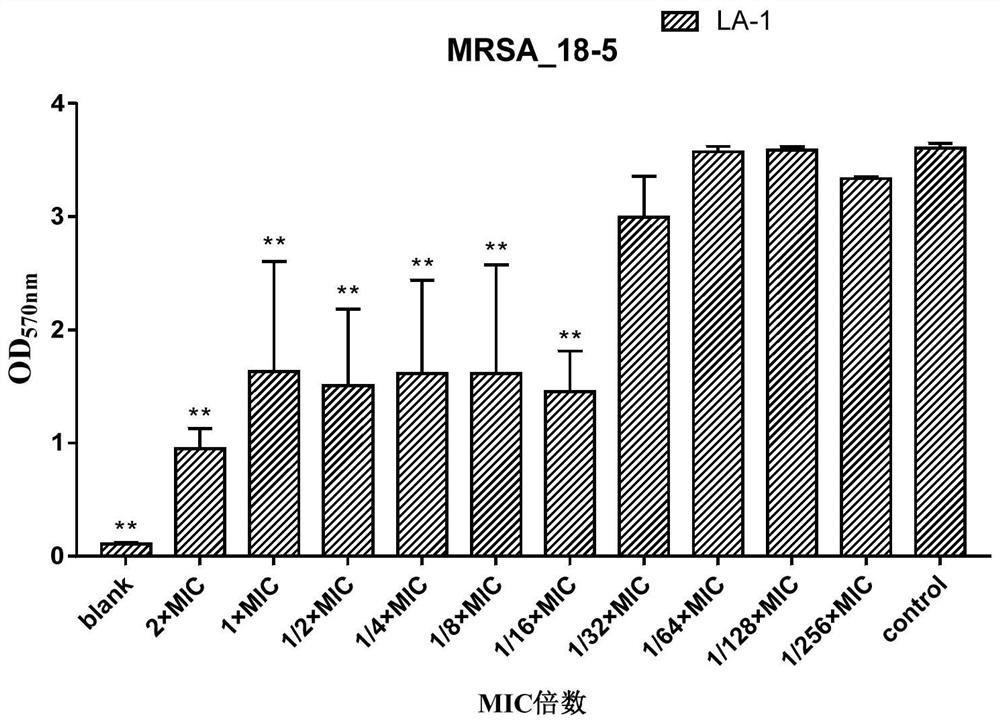
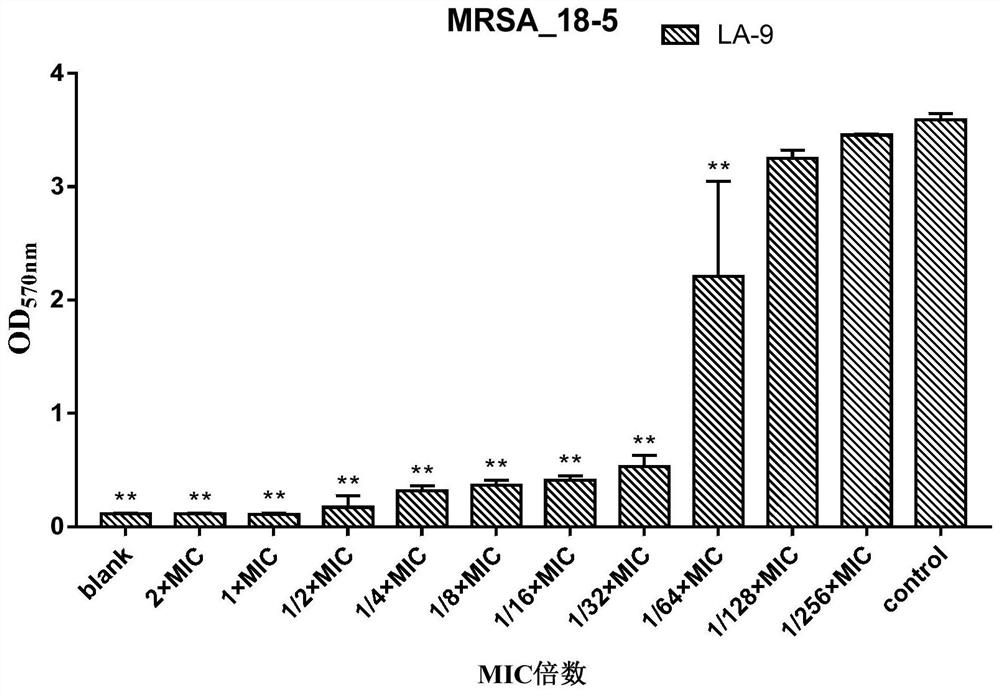
[0142] In this example, on the basis of Example 1 and Example 2, the inhibitory effect of compound LA-1 and compound LA-9 on methicillin-resistant Staphylococcus aureus (MRSA) biofilm formation was further investigated.
[0143] 1 Test product and reference product
[0144] 1.1 Test product
[0145] Table 9 information on the test product
[0146]
[0147] The information of the test products is shown in Table 9. Before use, the above test products were prepared with sterilized deionized water to a maximum concentration of 128ug / ml, and then double-diluted sequentially.
[0148] 1.2 Reference substance
[0149] Name: Norvancomycin
[0150] Batch number: 130338-201704 Manufacturer: China National Institutes for Food and Drug Control
[0151] Appearance: white powder Specification: 150mg / bottle
[0152] Storage Conditions: Sealed in a cool, dry place shading.
[0153] 2 Test instruments and reagents
[0154] DensiCHEK TM Plus electronic turbidimeter (Merieux Diagnosti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com