Application of inosine in preparation of antitumor drug or antitumor drug composition
A composition and anti-tumor technology, applied in the field of biomedicine, can solve the problems of poor curative effect, failure to prevent the rapid development of cancer, etc., and achieve the effects of convenient oral use, enhanced anti-tumor therapeutic efficacy, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
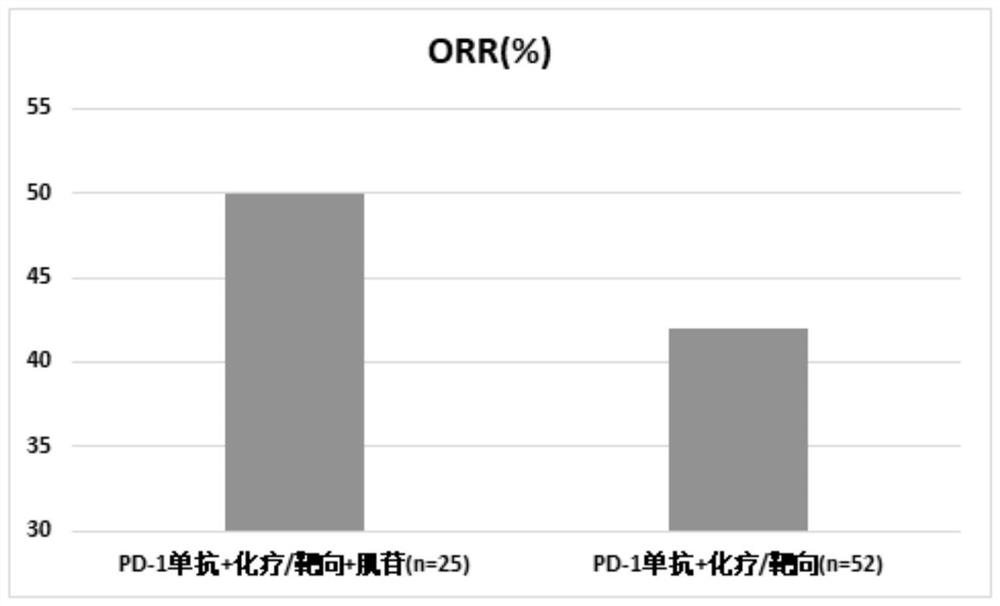
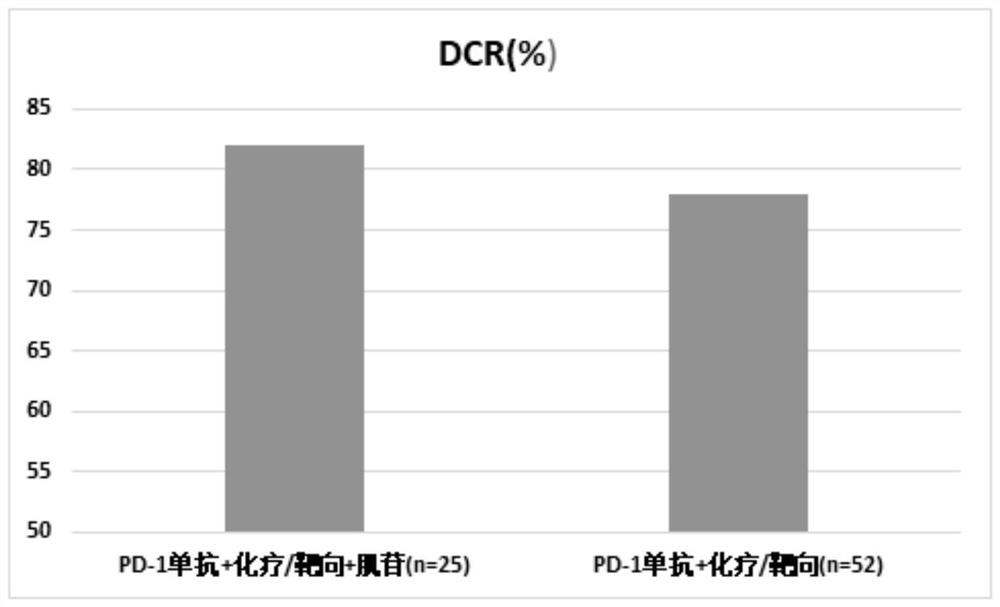
[0066] Clinical research on inosine combined with PD-1 monoclonal antibody and chemotherapy / targeted therapy:
[0067] 1. Case selection
[0068] Patients with advanced malignant solid tumors who were treated in the Oncology Department of Beijing Friendship Hospital between October 2020 and July 2021, this study has been approved by the Ethics Committee of Beijing Friendship Hospital.
[0069] 2. Drug information
[0070] Inosine tablets are provided by Guangdong Hengjian Pharmaceutical Co., Ltd., the specification is 0.2g*100 tablets, the batch number is H44021166, and the experimental drugs meet the treatment standards for clinical research;
[0071] PD-1 monoclonal antibody (Erica) is provided by Suzhou Centia Biomedical Co., Ltd., the specification is 200mg / bottle, the batch number is s20190027, and the experimental drug meets the treatment standards for clinical research;
[0072] The chemotherapeutic drug is: Ayue (paclitaxel albumin-bound type for injection), provided...
Embodiment 2
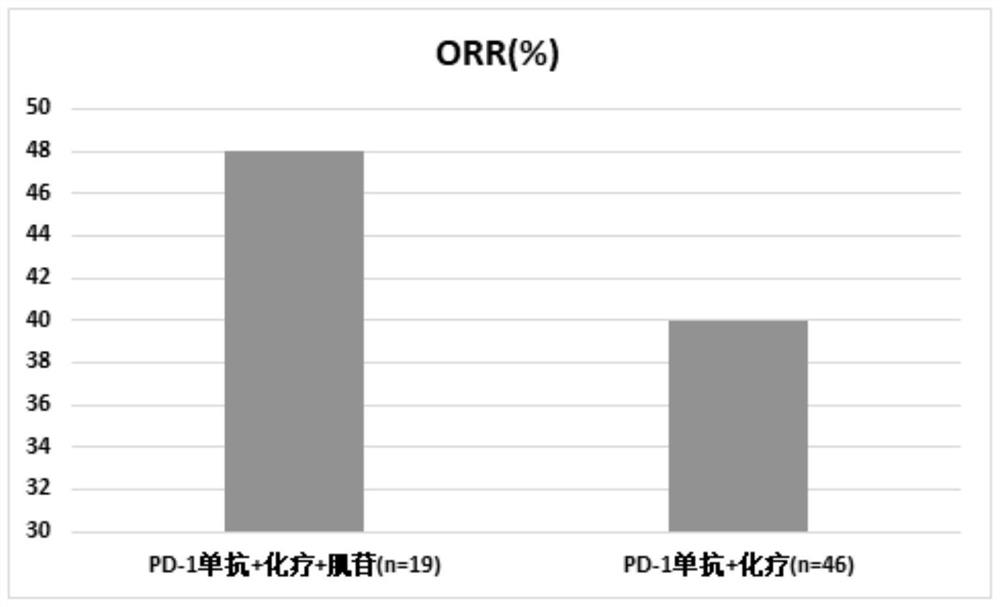
[0089] Clinical research on inosine combined with PD-1 monoclonal antibody and chemotherapy:
[0090] 1. Case selection
[0091] Patients with advanced malignant solid tumors who were treated in the Oncology Department of Beijing Friendship Hospital between October 2020 and July 2021, this study has been approved by the Ethics Committee of Beijing Friendship Hospital.
[0092] 2. Drug information
[0093] Inosine tablets are provided by Guangdong Hengjian Pharmaceutical Co., Ltd., the specification is 0.2g*100 tablets, the batch number is H44021166, and the experimental drugs meet the treatment standards for clinical research;
[0094] PD-1 monoclonal antibody: Erica, provided by Suzhou Centia Biomedicine Co., Ltd., the specification is 200mg / bottle, the batch number is s20190027, and the experimental drug meets the treatment standards for clinical research;
[0095] The chemotherapeutic drug is: Ayue (paclitaxel albumin-bound type for injection), provided by Jiangsu Hengrui...
Embodiment 3
[0110] Clinical research on inosine combined with PD-1 monoclonal antibody and targeted therapy:
[0111] 1. Case selection
[0112] Patients with advanced malignant solid tumors who were treated in the Oncology Department of Beijing Friendship Hospital between October 2020 and July 2021, this study has been approved by the Ethics Committee of Beijing Friendship Hospital.
[0113] 2. Drug information
[0114] Inosine tablets are provided by Guangdong Hengjian Pharmaceutical Co., Ltd., the specification is 0.2g*100 tablets, the batch number is H44021166, and the experimental drugs meet the treatment standards for clinical research;
[0115] PD-1 monoclonal antibody (Erica) is provided by Suzhou Centia Biomedical Co., Ltd., the specification is 200mg / bottle, the batch number is s20190027, and the experimental drug meets the treatment standards for clinical research;
[0116] Anlotinib was provided by Chia Tai Tianqing Co., Ltd., with a specification of 8 mg, batch number 19092...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com