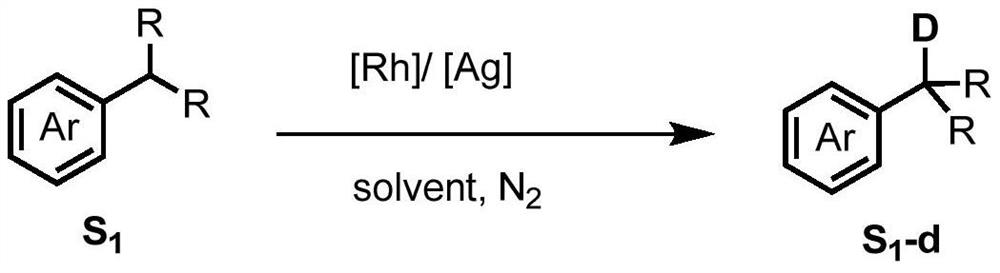
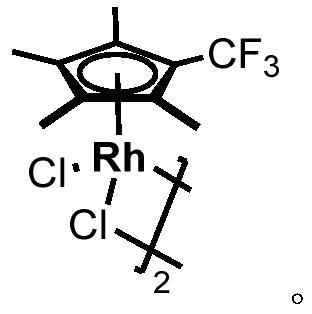
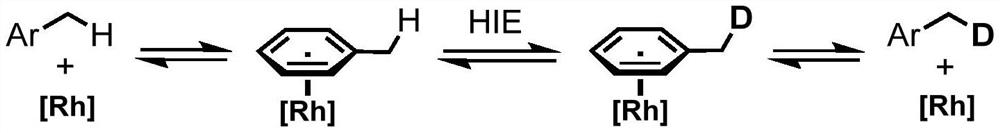
Method for selective deuteration of aromatic ring benzyl carbon-hydrogen bonds
An aromatic ring benzylic position and selective technology, applied in the field of hydrogen-deuterium exchange, can solve the problems of poor deuterium substitution selectivity and narrow substrate applicability, and achieve the effects of simple operation, convenient operation and handling, and wide compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] In a nitrogen atmosphere, sequentially add raw material 1 (0.2mmol, 30.0mg), catalyst 10 (0.005mmol, 3.5mg), AgNTf 2 (0.02mmol, 7.8mg), Li 3 PO 4 (0.02mmol, 2.3mg), finally add acetone-d 6 (0.2 mL). After the reaction was stirred at 120°C for 24 hours, it was cooled to room temperature, and the internal standard 1,1,2,2-tetrachloroethane (0.2mmol, 33.6mg) and deuterated chloroform (0.5mL) were added, and determined by H NMR spectroscopy. The benzylic deuterated rate of the target product was 86%, and the NMR yield was 99%. 1 H NMR (500MHz, Chloroform-d) δ7.16(d, J=8.7Hz, 2H), 6.85(d, J=8.7Hz, 2H), 3.80(s, 3H), 2.92–2.80(m, 0.14H ,86%D), 1.28–1.16(m,6H). 13 C NMR(126MHz,Chloroform-d)δ157.6,127.2,141.0,113.7,55.2,33.2(benzylic carbon of remaining1),32.8(t,J=19.5Hz,benzylic carbon of deuterated 1),24.19–24.08(m,– CH 3 carbon).
Embodiment 2
[0038]
[0039] In a nitrogen atmosphere, sequentially add raw material 2 (0.2mmol, 24.0mg), catalyst 1 (0.005mmol, 3.1mg), AgOTf (0.02mmol, 5.1mg), NaOTf (0.2mmol, 34.4mg), and finally add methanol-d 4 (0.2 mL). After the reaction was stirred at 120°C for 24 hours, it was cooled to room temperature, and the internal standard 1,1,2,2-tetrachloroethane (0.2mmol, 33.6mg) and deuterated chloroform (0.5mL) were added, and determined by H NMR spectroscopy. The benzylic deuterated rate of the target product was 50%, and the NMR yield was 99%. 1 H NMR (500MHz, Chloroform-d) δ7.30–7.26(m,2H),7.23–7.21(m,2H),7.19–7.15(m,1H),2.95–2.86(m,0.50H,50%D ),1.26–1.21(m,6H). 13 C NMR(126MHz,Chloroform-d)δ148.74–148.71(m,aromatic carbon adjacent to benzylic carbon),128.2,126.3,125.6,34.0(benzylic carbon of remaining 2),33.53(t,J=19.5Hz,benzylic carbon of deuterated 2),23.81–23.70(m,–CH 3 carbon).
Embodiment 3
[0041]
[0042] In a nitrogen atmosphere, sequentially add raw material 3 (0.2mmol, 29.7mg), catalyst 11 (0.005mmol, 3.7mg), AgNTf 2 (0.02mmol, 7.8mg), NaNTf 2 (0.2mmol, 60.6mg), finally add methanol-d 4 (0.2 mL). After the reaction was stirred at 120°C for 24 hours, it was cooled to room temperature, and the internal standard 1,1,2,2-tetrachloroethane (0.2mmol, 33.6mg) and deuterated chloroform (0.5mL) were added, and determined by H NMR spectroscopy. The benzylic deuterated rate of the target product was 83%, and the NMR yield was 99%. 1 H NMR (500MHz, Chloroform-d) δ7.31 (d, J = 8.3Hz, 2H), 7.14 (d, J = 8.3Hz, 2H), 2.38–2.31 (m, 0.51H, 83%D), 1.34 (s,6H). 13 C NMR(126MHz,Chloroform-d)δ148.2,134.8–134.7(m,aromatic carbon adjacent to benzylic methyl carbon),128.7,125.1,34.3,31.4,20.8–19.4(m,benzylic methyl carbon).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com