Recombinant gene, vector and exosome for double-way antagonism of PCSK9 as well as preparation method and application of exosome
A technology for recombining genes and exosomes, applied in the field of genetic engineering, can solve the problems of limited antagonism efficiency, adverse reactions, and high production costs, and achieve the effects of low cost, reduced degradation, and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a method for preparing the above-described exosomes, comprising the steps of:
[0029] After the above-described recombinant expression vector transfected into human mesenchymal stem cells (the MSC), incubated to obtain exosomes, namely EGFA modified exosomes.
[0030] In the present invention, comprising the transfection of the recombinant expression vector and transfection reagent mixing and the MSC, the recombinant expression vector mass volume and transfection reagent is preferably from (2 ~ 4) μg: (4 ~ 8 ) μL, more preferably (2.5 ~ 3.5) μg: (5 ~ 7) μL.
[0031] In the present invention, the incubation is preferably carried out in a cell incubator; the incubation temperature is preferably 37 [deg.] C; during incubation, CO 2 The volume percentage of preferably 5%; incubating the incubation comprises a first and a second incubation; preferably in the absence of the first double-antibody incubation medium (DMEM, Gibco, USA; FBS, Gibco compa...
Embodiment 1
[0039] Construction of recombinant expression vector: the recombinant expression vector is done by Nanjing GenScript Corporation;
[0040] (1) selection of restriction sites XhoI and HindⅢ;
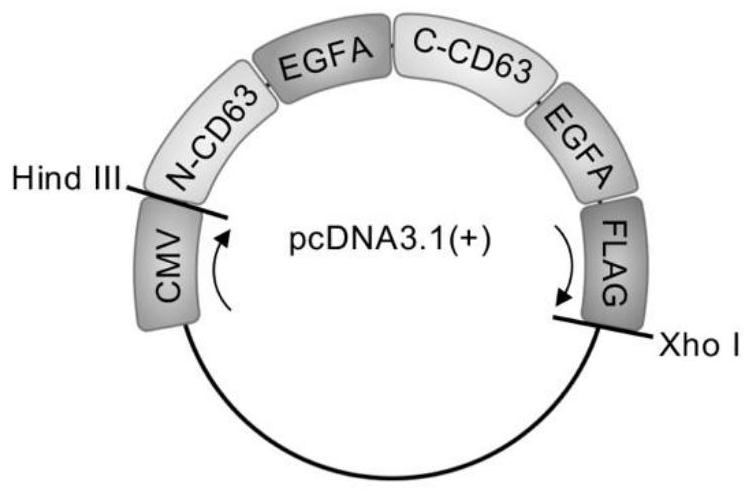
[0041] (2) 3 'end to the 5' end is connected Kozak sequence fragment (as shown in SEQ ID No.3), CD63 first segment (e.g. as shown in SEQ ID No. 4), outer-EGF-A fragment (e.g., SEQ FIG ID No.5), CD63 fragment of a second gene (e.g., SEQ IDNo.6 shown), inner-EGF-A fragment (as shown in SEQ ID) and No.7 FLAG tag (e.g., as SEQ ID No. 8 shown), giving a recombinant gene, the nucleotide sequence shown in sequence Listing SEQ ID Nos. 1;
[0042] (3) The above recombinant gene loaded pcDNA3.1 (+) and XhoⅠ HindⅢ between restriction sites vector successfully humanized CD63-2 × EGF-A motif loaded into pcDNA3.1 (+) on a support , recombinant expression vectors, e.g. figure 1 Indicated.
[0043] (4) 2 ~ 4μg recombinant expression vector with 4 ~ 8μL after transfection reagent mixture, after standing for 3...
Embodiment 2
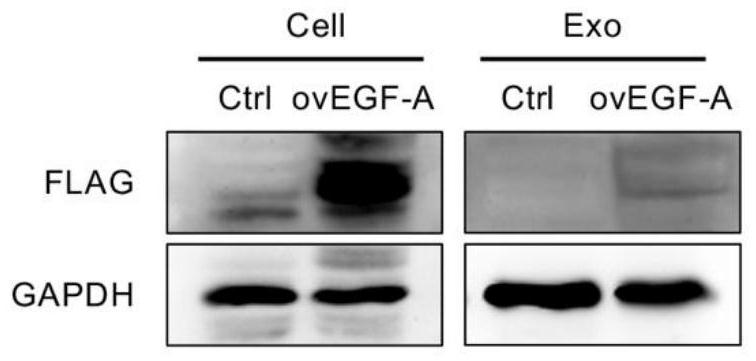
[0049] The resulting secreted outside the body westernblot detecting FLAG tag.
[0050] The extraction method of the exosomes precipitate after centrifugation exosomes, vacuum suction residual liquid was added 50μL RIPA lysis buffer (Pik days, China), by pipetting with a pipette to completely dissolve exosomes, placed on ice 30min, so that sufficient exosomes proteins cleavage. For protein quantification (Thermo, USA) by doubling dilution and protein sample preparation. 12% of the gel formulation itself (Pik days, China), was added after exosomes protein samples were electrophoresed. Setting electrophoresis voltage 90V, 30min, start electrophoresis. When the protein samples into the underlying separation gel, the electrophoresis voltage is adjusted to 120V, 90min. Protein samples were observed position when the position reaches the electrophoresis can be stopped, in turn be transferred to a membrane, to set current 200mA, expected time 120min. After antibody incubation. The antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com