Polyethyleneimine derivative as well as preparation method and application thereof
A technology of polyethyleneimine and its derivatives, applied in the field of biomedicine, can solve the problems of limiting PEI, low loading efficiency, unstable loading, etc., and achieve the effect of reducing cytotoxicity and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
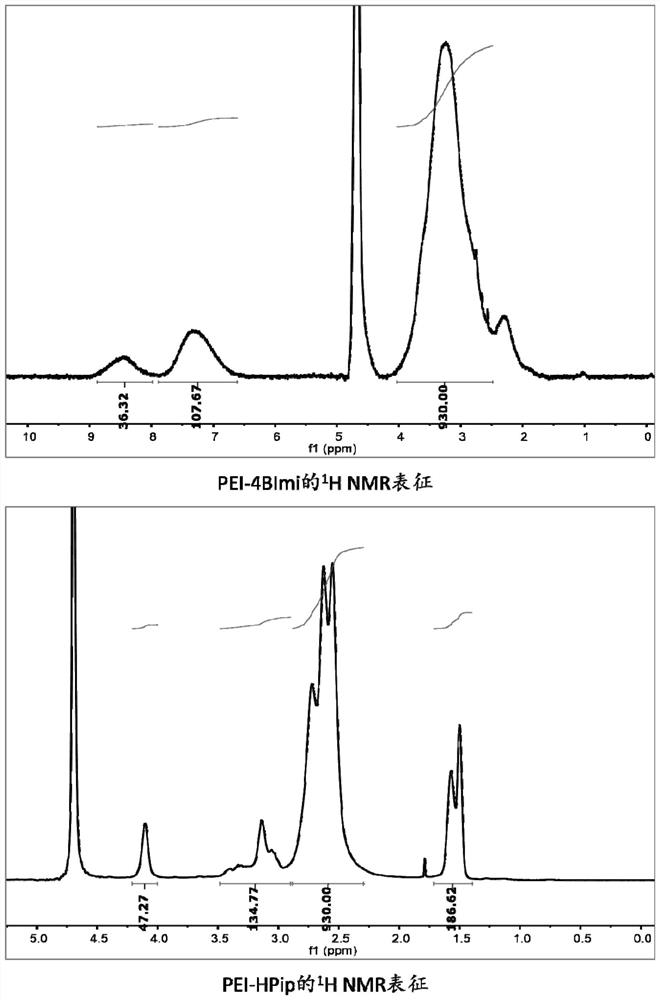
[0055] The preparation of hyperbranched PEI material (PEI-M) with five-membered ring and six-membered ring modification, 40 times equivalent of five-membered and six-membered nitrogen-containing heterocyclic small molecules (as shown in Table 2), 60 times equivalent of EDC Weigh HCl, 80 times the equivalent of NHS into a reaction bottle with a stirring bar, add anhydrous DMSO and stir the reaction at 35°C for 30 minutes to activate the carboxyl group on the small molecule, then add 1 equivalent of it that has been dissolved in DMSO The hyperbranched PEI-10kDa reaction was continued with stirring at 35 °C for 72 h. Subsequently, the reaction system was settled with 10 times the volume of diethyl ether, the upper layer liquid was discarded, and the residual diethyl ether was sucked dry. Then dissolve the reaction product with sterile water, dialyze in Milli Q water with a dialysis bag with a molecular weight cut-off of 7000Da, the pH value gradient increases from 3-7, change the...
Embodiment 2
[0061] The preparation of the hyperbranched PEI material (PEI-M) modified by the seven-membered ring, 48 times the equivalent of carbonyldiimidazole (CDI) was weighed into a reaction bottle with a stirring bar, dissolved in anhydrous DMSO, and 40 times the equivalent of seven Small nitrogen-containing heterocyclic molecules (as shown in Table 2), specifically N-(2-hydroxyethyl)hexamethylenediamine, were injected into the reaction vial with a syringe, and then stirred and reacted at 35°C for 12 hours to carry out the hydroxyl group reaction. For the reaction with CDI, add 1 equivalent of hyperbranched PEI-10kDa dissolved in DMSO in advance, and continue to stir and react at 35°C for 24 hours. Subsequently, the reaction system was settled with 10 times the volume of diethyl ether, the upper layer liquid was discarded, and the residual diethyl ether was sucked dry. Then dissolve the reaction product with sterile water, and dialyze in MilliQ water with a dialysis bag with a molecu...
Embodiment 3
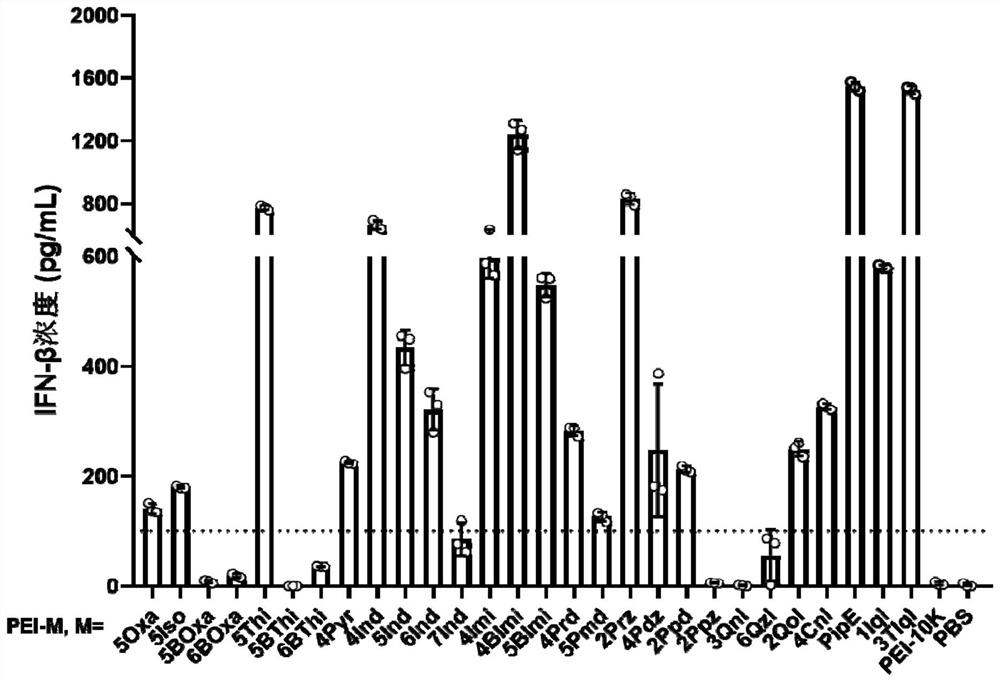
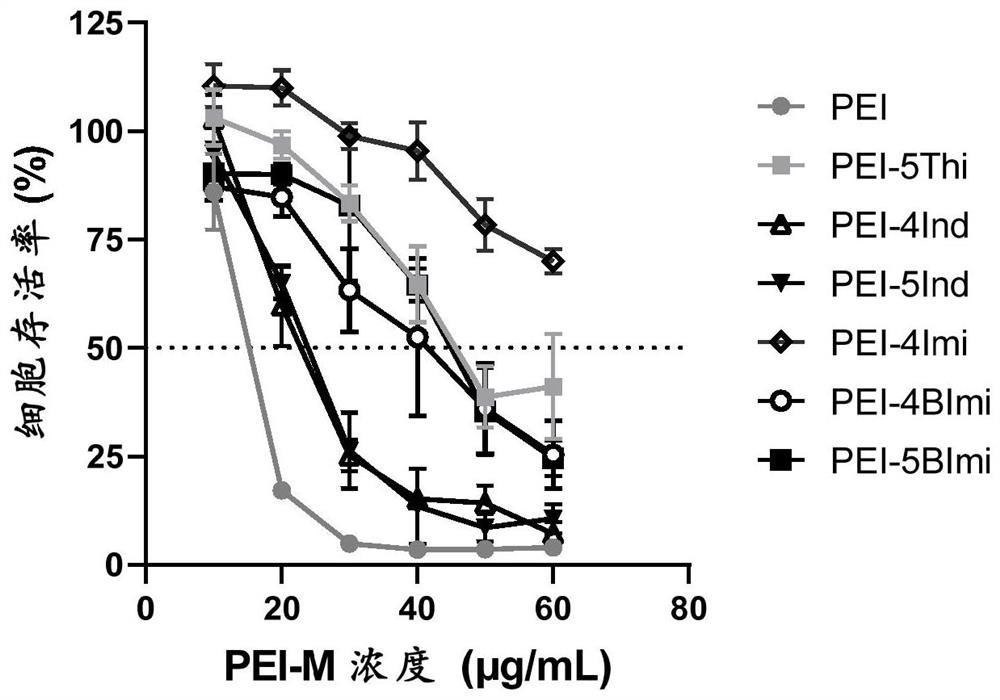
[0064] The 28 kinds of PEI-M obtained in Example 1 were used to induce DC2.4 cells to release IFN-β. Fully dissolve PEI-M with sterile water and add it to a 96-well plate with 15,000 DC2.4 cells per well. The concentration of PEI-M is 20 μg / mL or 50 μg / mL. The volume of PEI-M-containing medium was 200 μL. After 24 hours, the medium in the well plate was collected and centrifuged at 3000 rpm for 5 minutes, the medium supernatant was collected, and the concentration of IFN-β was detected with an ELISA kit. The process of ELISA detection is (all processes are carried out at room temperature): incubate the capture antibody overnight in a high-adhesion 96-well plate, wash three times with PBST (containing 0.05% PBS solution), block with PBS containing 1% BSA for 1 hour, PBST Wash 3 times, add the sample to be detected (3-4 replicates) and Bracket sample and incubate for 2 hours, wash 3 times with PBST, add detection antibody and incubate for 2 hours, wash 3 times with PBST, add st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com