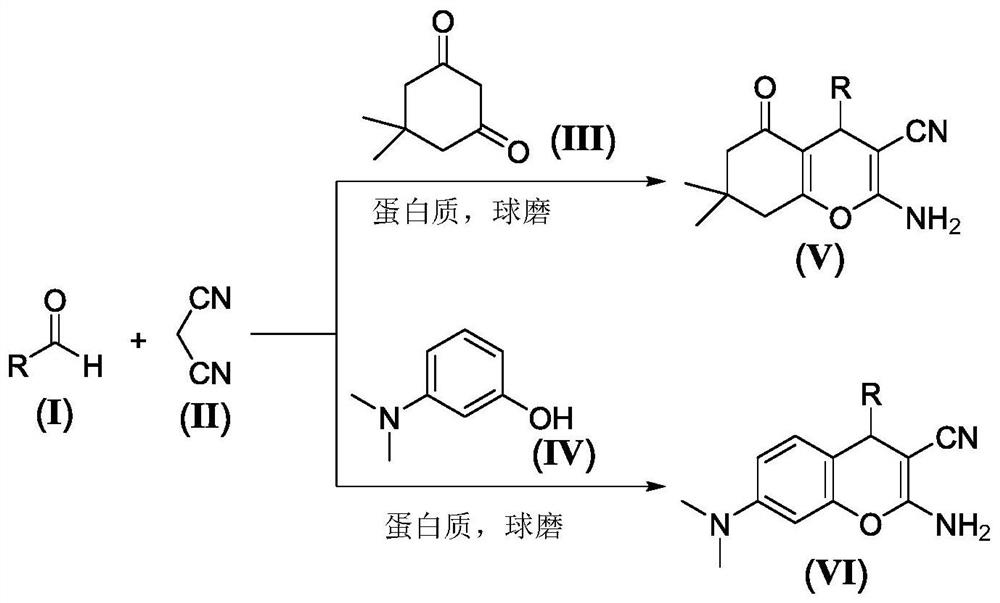
Mechanical ball milling assisted synthesis method of 2-amino-3-cyano-4H-pyran compound
A technology of mechanical ball milling and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of heating synthesis time, tedious catalyst preparation, etc., achieve short reaction time, realize green synthesis, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
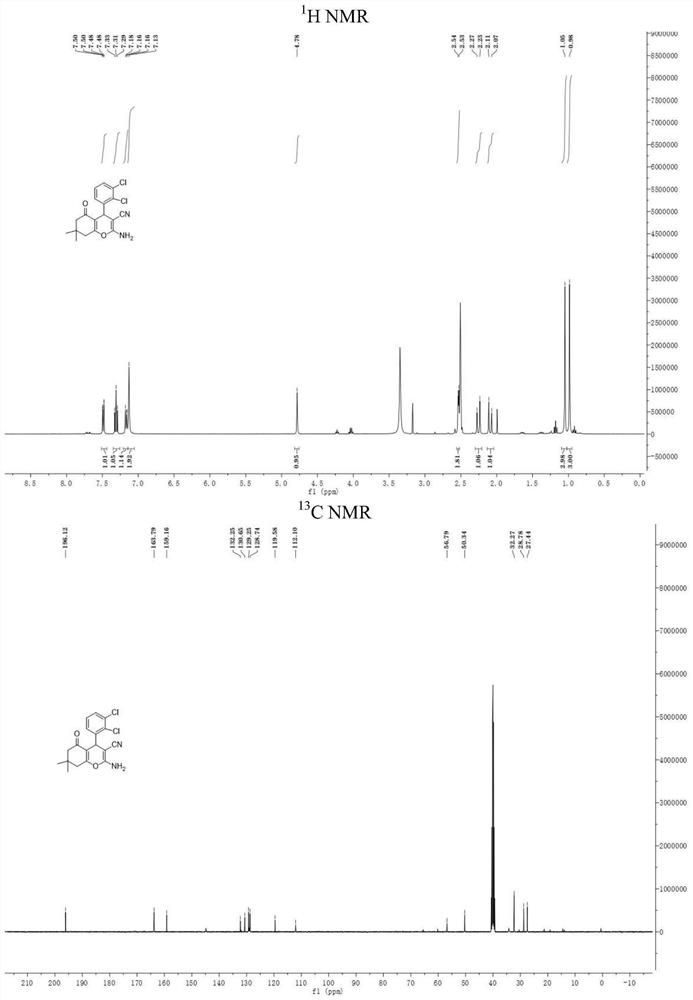
Image
Examples
Embodiment 1
[0034] (a) Three stainless steel balls with a diameter of 10 mm were added to a 50 mL stainless steel ball mill jar, and then 0.5 mmol of benzaldehyde, 0.5 mmol of malononitrile, 0.5 mmol of dimedone, 50 mg of bovine serum albumin and 300 mg of Sodium chloride, ball milled for 30 minutes at 20Hz;
[0035] (b) After ball milling, scrape off the reaction mixture, add 30 mL of ethyl acetate to dissolve and filter, take the filtrate to concentrate by rotary evaporation, and finally purify by column chromatography, the eluent used is a mixed solution of petroleum ether and ethyl acetate , the volume ratio of sherwood oil and ethyl acetate is 4:1;
[0036] (c) The eluate containing the product was rotary evaporated to obtain 2-amino-3-cyano-4-phenyl-7,7-dimethyl-5-oxo-4H-5,6, 7,8-tetrahydrobenzo[b]pyran, yield 72.8%.
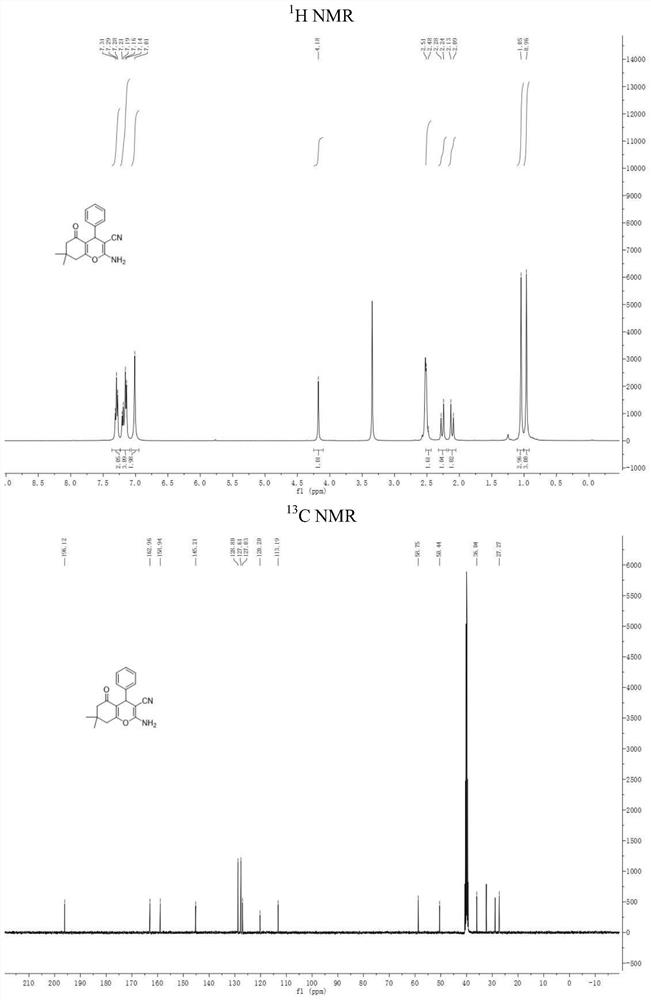
[0037] The H NMR and C NMR spectra of the product are as follows: figure 2 As shown, its characterization data is m. p. 222-223 ℃, 1 HNMR (400 MHz, DMSO- d 6 )...
Embodiment 2
[0039](a) Three stainless steel balls with a diameter of 10 mm were added to a 50 mL stainless steel ball mill jar, and then 0.5 mmol of benzaldehyde, 0.5 mmol of malononitrile, 0.5 mmol of dimedone, 50 mg of bovine serum albumin and 300 mg of Sodium chloride, ball milled for 40 minutes at 30Hz;
[0040] (b) After ball milling, scrape off the reaction mixture, add 30 mL of ethyl acetate to dissolve and filter, take the filtrate to concentrate by rotary evaporation, and finally purify by column chromatography, the eluent used is a mixed solution of petroleum ether and ethyl acetate , the volume ratio of sherwood oil and ethyl acetate is 4:1;
[0041] (c) The eluate containing the product was rotary evaporated to obtain 2-amino-3-cyano-4-phenyl-7,7-dimethyl-5-oxo-4H-5,6, 7,8-Tetrahydrobenzo[b]pyran, the yield was 96.0%.
[0042] The characterization data of this product are the same as the product of Example 1.
Embodiment 3
[0044] (a) Three stainless steel balls with a diameter of 10 mm were added to a 50 mL stainless steel ball mill jar, followed by 0.5 mmol benzaldehyde, 0.5 mmol malononitrile, 0.5 mmol dimedone and 50 mg bovine serum albumin, 30 Hz Ball milling for 30 minutes;
[0045] (b) After ball milling, scrape off the reaction mixture, add 30 mL of ethyl acetate to dissolve and filter, take the filtrate to concentrate by rotary evaporation, and finally purify by column chromatography, the eluent used is a mixed solution of petroleum ether and ethyl acetate , the volume ratio of sherwood oil and ethyl acetate is 4:1;
[0046] (c) The eluate containing the product was rotary evaporated to obtain 2-amino-3-cyano-4-phenyl-7,7-dimethyl-5-oxo-4H-5,6, 7,8-Tetrahydrobenzo[b]pyran in 42.5% yield.
[0047] The characterization data of this product are the same as the product of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com