Polypeptide derivative with dual-receptor excitement effect and application of polypeptide derivative
A technology of peptide derivatives and derivatives, applied in the field of medicine and biology, can solve problems such as no drugs on the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] The basic linear sequence of the polypeptide provided by the present invention and the side chain modified derivative peptide are prepared according to the following general methods:
[0153] 1) Synthesis: Using the Fmoc strategy, use the PSI200 peptide synthesizer to synthesize step by step according to the following steps:
[0154] a) In the presence of an activator system, the Fmoc-amino acid-resin is obtained by coupling the resin solid phase carrier and the Fmoc-protected C-terminal amino acid; wherein, the synthesis of the C-terminal amidated polypeptide uses an amino resin, such as Rink Amide AM, Rink Amide , Rink MBHA, etc.; the Fmoc-amino acid and resin ratio (mol / mol) is 3 to 5:1, and the coupling activator is HOBT / DIC or HOBT / HBTU / DIEA.
[0155] b) Extension of the peptide chain: link amino acids in accordance with the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain a peptide-resin conjugate with N-terminal and side chain protec...
Embodiment 2
[0167] Example 2 Effect on GLP-1 / GC receptor
[0168] The effect of the polypeptides on the GLP-1 / GC receptor was evaluated by the effect on GLP-1 / GC receptor-mediated cAMP production in vitro.
[0169] HEK293 cell lines stably expressing GLP-1R and GCGR are used for screening of GLP-1R agonists and GCGR agonists.
[0170] Test sample preparation: the compound was formulated into a 100μM stock solution, and gradually diluted to 1μM, 100nM, 10nM, 1nM, 10 -1 nM, 10 -2 nM, 10 -3 nM, H 2 O working concentration.
[0171] Positive control substance preparation:
[0172] GLP-1:
[0173] Storage method: 1mM storage solution dissolved in 0.1% BSA ultrapure water, and stored at -80°C.
[0174] Working concentration: 1μM, 100nM, 10nM, 1nM, 10 -1 nM, 10 -2 nM, 10 -3 nM and H 2 O (each well contains 0.1% BSA).
[0175] Glucagon (GC):
[0176] Storage method: 1mM DMSO solution, store in airtight at -80°C.
[0177] Working concentration: 1μM, 100nM, 10nM, 1nM, 10 -1 nM, 10 ...
Embodiment 3
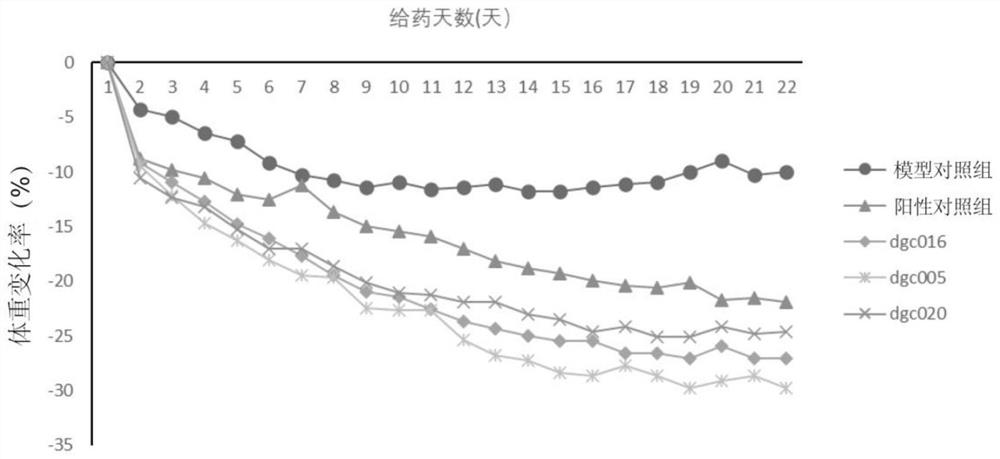
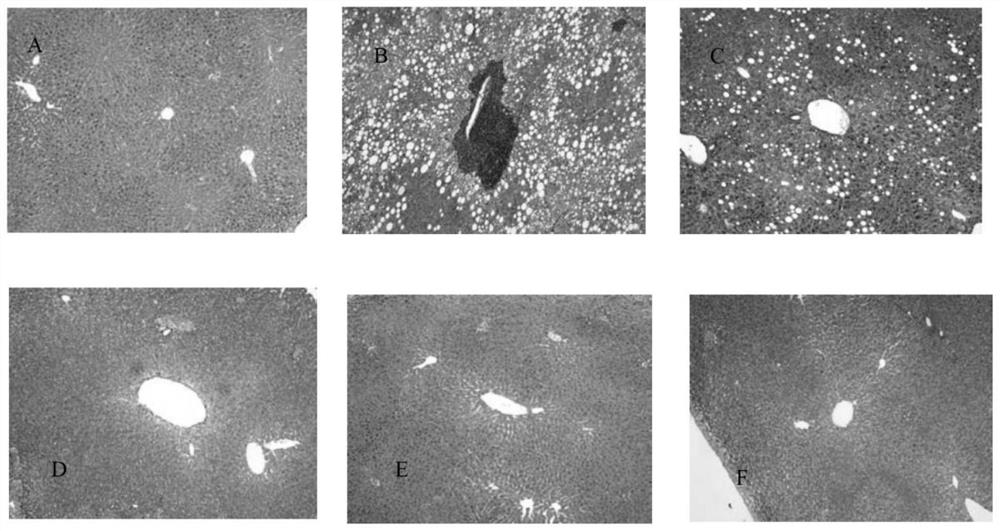
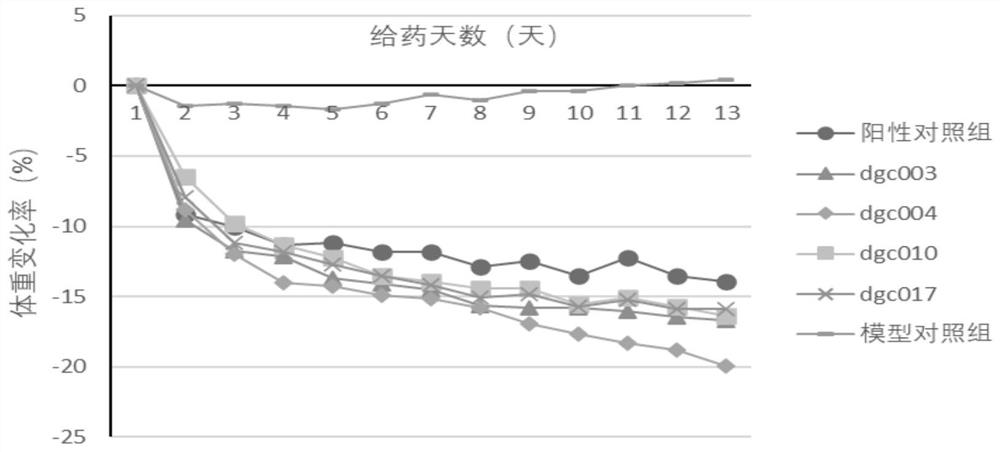
[0192] Example 3 The hypoglycemic effect of embodiment 1 compound
[0193] The hypoglycemic effect of the compound of Example 1 was evaluated by a single-administration glucose load test in normal mice.
[0194] Methods: Kunming mice (male, body weight range 20-22g) were adaptively fed in the barrier environment animal room for 3 days and then divided into blank control group, model control group, positive control group and test compound group, with 5 mice in each group , fasting 6h. The blank control group and the model control group were given physiological saline, the dose of the test compound group and the positive control group was 30 nmol / kg, and the positive control drug was semaglutide. After 60min of administration, the model control group, the positive control group and the test compound group were treated with 2.5g / kg (0.01mL·g -1 Body weight) were given glucose by intragastric administration, and the blank control group was given distilled water, and the blood ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com